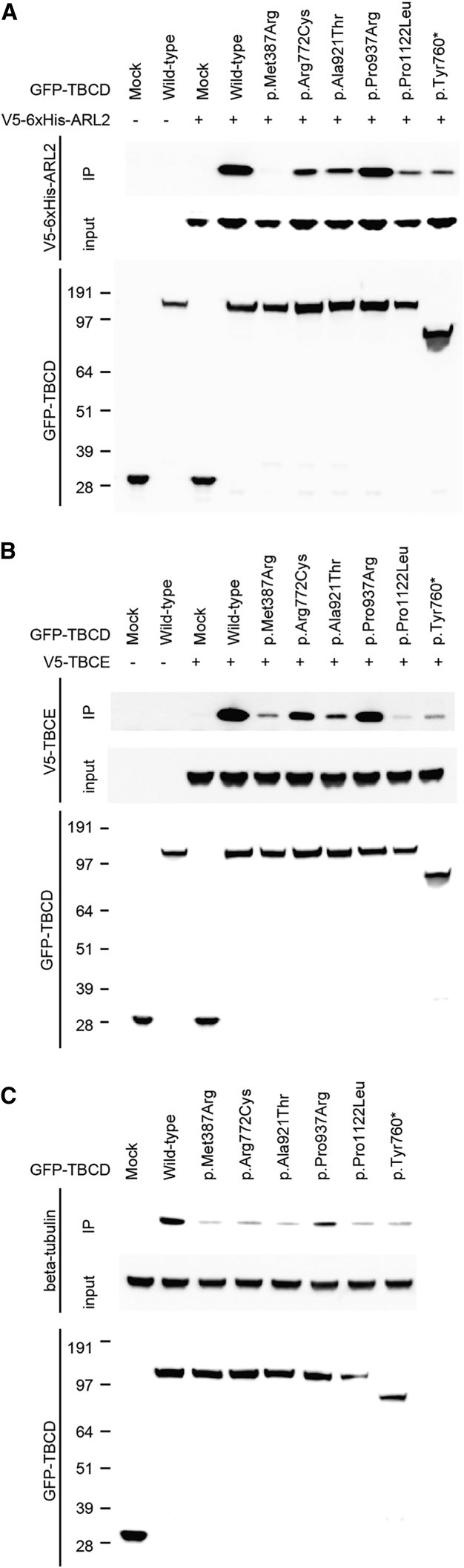
Figure 4.
Intermolecular Associations between TBCD and Its Binding Partners
(A and B) The molecular interaction between human TBCD and ARL2 (A) or TBCE (B). GFP-fused wild-type TBCD (159 kDa) or its mutants were transiently co-overexpressed with V5-6xHis-tagged ARL2 (22 kDa) or V5-tagged TBCE (60 kDa) in HEK293T cells using the X-treme GENE9 DNA transfection kit (Roche). At 48 hr after transfection, the cells were collected and lysed with lysis buffer (25 mM Tris-HCl [pH 7.5], 100 mM NaCl, 2 mM EDTA, 0.5% Triton X-100, and protease inhibitors). GFP-fused TBCD proteins were immunoprecipitated using a rabbit polyclonal anti-GFP antibody (Abcam cat# ab6556, RRID: AB_305564) and Dynabeads Protein-G (Thermo Fisher Scientific). Input and immunoprecipitated samples (IP) were immunoblotted with mouse monoclonal anti-GFP (Roche) and anti-V5 antibodies (Thermo Fisher Scientific cat# R960-25, RRID: AB_2556564).
(C) The binding of human TBCD and endogenous β-tubulin (50 kDa). Wild-type GFP-fused TBCD or its mutants were transiently overexpressed in HEK293T cells. GFP-fused TBCD proteins were immunoprecipitated with a rabbit polyclonal anti-GFP antibody and immunoblotted with mouse monoclonal anti-GFP or anti β-tubulin antibodies (Chemicon International cat# MAB3408). Horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch) was used as the secondary antibody. Immunodetection was performed using Supersignal West Dura Extended Duration Substrate (Thermo Fisher Scientific) on a ChemiDoc Touch Imaging system (Bio-Rad Laboratories).
At least three independent experiments were performed to confirm the consistency of results for TBCD binding with ARL2, TBCE, or β-tubulin. Mock indicates only GFP protein (27 kDa). “+” and “−” denote the presence and absence of V5-tagged proteins (or only V5-tag with the size of 1 kDa, which could not be detected due to its small molecular weight), respectively.