Abstract
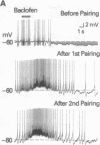
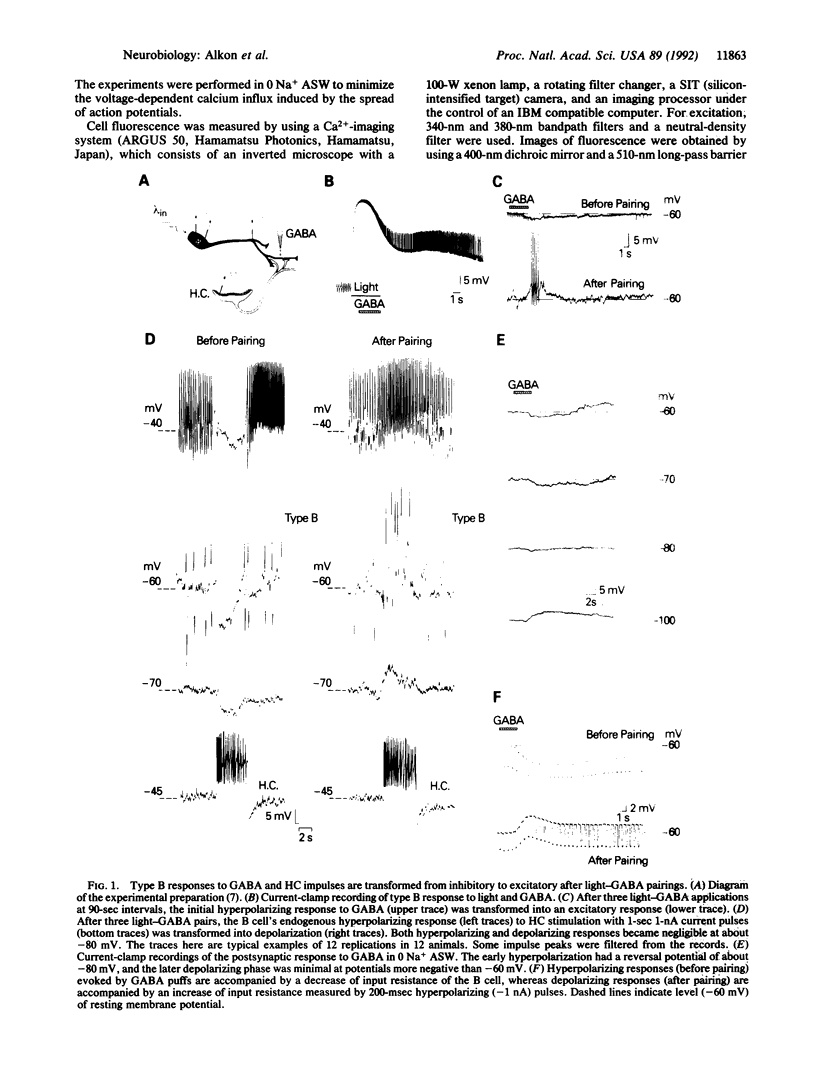
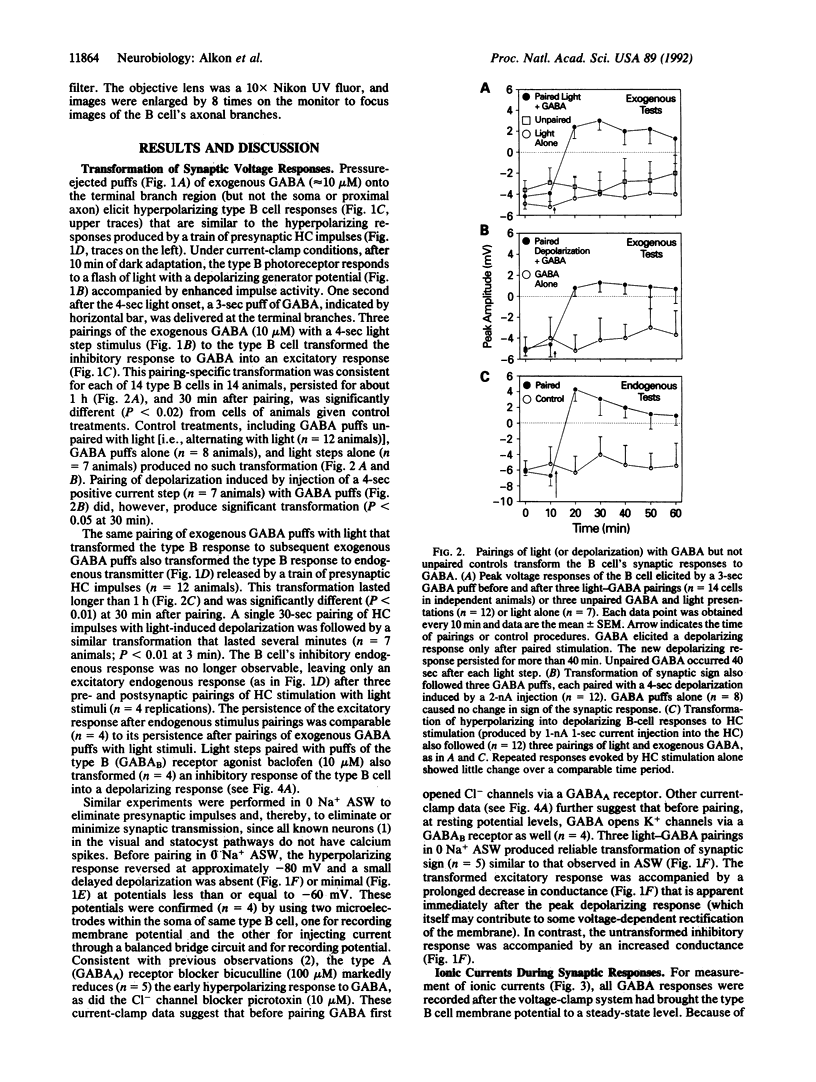
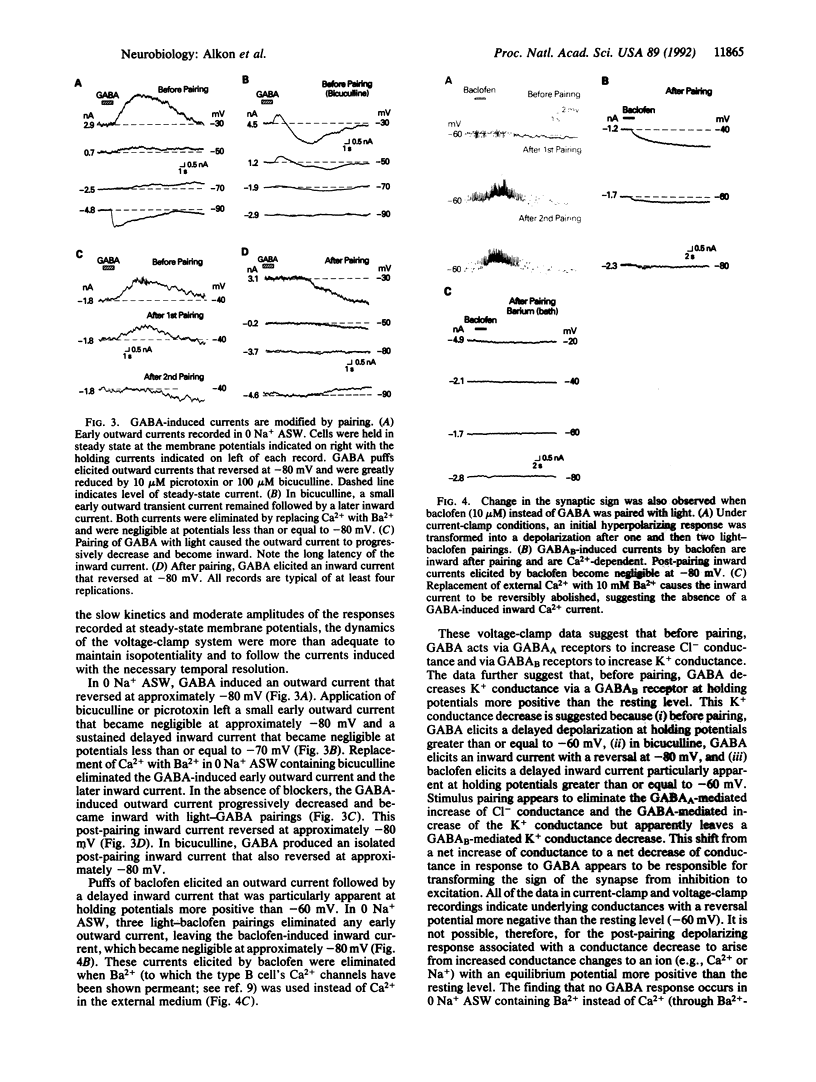
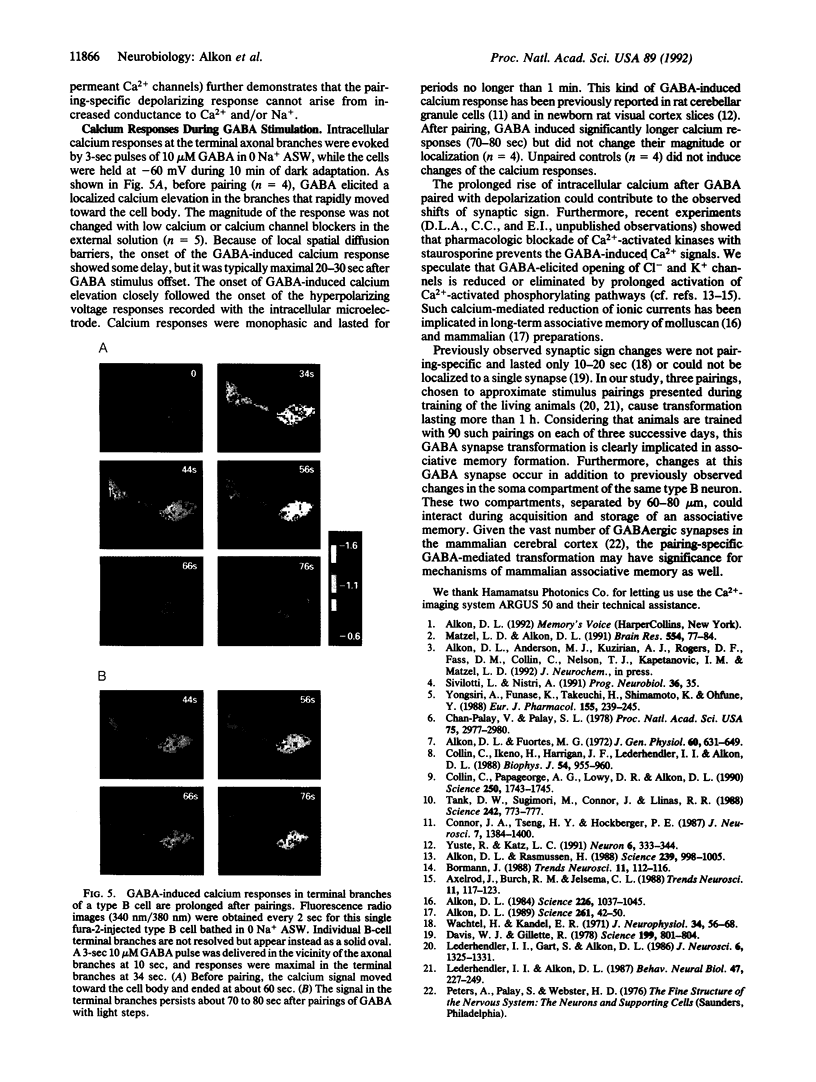
For a constant membrane potential, a predominantly inhibitory GABAergic synaptic response is shown to undergo long-term transformation into an excitatory response after pairing of exogenous gamma-aminobutyric acid (GABA) with postsynaptic depolarization or pairing of pre- and postsynaptic stimulation. Current- and voltage-clamp experiments suggest that this synaptic transformation is due to a shift from a net increase of conductance to a net decrease of conductance in response to GABA. GABA-induced elevation of intracellular calcium is prolonged after the same stimulus pairing and may, therefore, contribute to this synaptic transformation via Ca(2+)-activated phosphorylation pathways. This synaptic transformation, which does not follow unpaired stimulus presentations, occurs in a neuronal compartment spatially separated from the soma, which also changes during stimulus pairing.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akon D. L., Fuortes M. G. Responses of photoreceptors in Hermissenda. J Gen Physiol. 1972 Dec;60(6):631–649. doi: 10.1085/jgp.60.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Alkon D. L. Memory storage and neural systems. Sci Am. 1989 Jul;261(1):42–50. doi: 10.1038/scientificamerican0789-42. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Ultrastructural localization of gamma-aminobutyric acid receptors in the mammalian central nervous system by means of [3H]muscimol binding. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2977–2980. doi: 10.1073/pnas.75.6.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Ikeno H., Harrigan J. F., Lederhendler I., Alkon D. L. Sequential modification of membrane currents with classical conditioning. Biophys J. 1988 Nov;54(5):955–960. doi: 10.1016/S0006-3495(88)83031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Papageorge A. G., Lowy D. R., Alkon D. L. Early enhancement of calcium currents by H-ras oncoproteins injected into Hermissenda neurons. Science. 1990 Dec 21;250(4988):1743–1745. doi: 10.1126/science.2176747. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. J., Gillette R. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science. 1978 Feb 17;199(4330):801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- Lederhendler I. I., Alkon D. L. Associatively reduced withdrawal from shadows in Hermissenda: a direct behavioral analog of photoreceptor responses to brief light steps. Behav Neural Biol. 1987 May;47(3):227–249. doi: 10.1016/s0163-1047(87)90370-0. [DOI] [PubMed] [Google Scholar]
- Lederhendler I. I., Gart S., Alkon D. L. Classical conditioning of Hermissenda: origin of a new response. J Neurosci. 1986 May;6(5):1325–1331. doi: 10.1523/JNEUROSCI.06-05-01325.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel L. D., Alkon D. L. GABA-induced potentiation of neuronal excitability occurs during contiguous pairings with intracellular calcium elevation. Brain Res. 1991 Jul 19;554(1-2):77–84. doi: 10.1016/0006-8993(91)90174-t. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]
- Yongsiri A., Funase K., Takeuchi H., Shimamoto K., Ohfune Y. Classification of GABA receptors in snail neurones. Eur J Pharmacol. 1988 Oct 18;155(3):239–245. doi: 10.1016/0014-2999(88)90509-2. [DOI] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]