Abstract
The biosynthesis of Fe-S clusters in Bacillus subtilis and other Gram-positive bacteria is catalyzed by the SufCDSUB system. The first step in this pathway involves the sulfur mobilization from the free amino acid cysteine to a sulfur acceptor protein SufU via a PLP-dependent cysteine desulfurase SufS. In this reaction scheme, the formation of an enzyme S-covalent intermediate is followed by the binding of SufU. This event leads to the second half of the reaction where a deprotonated thiol of SufU promotes the nucleophilic attack onto the persulfide intermediate of SufS. Kinetic analysis combined with spectroscopic methods identified that the presence of a zinc atom tightly bound to SufU (Ka=1017 M−1) is crucial for its structural and catalytic competency. Fe-S cluster assembly experiments showed that despite the high degree of sequence and structural similarity to the ortholog enzyme IscU, the B. subtilis SufU does not act as a standard Fe-S cluster scaffold protein. The involvement of SufU as a dedicated agent of sulfur transfer, rather than as an assembly scaffold, in the biogenesis of Fe-S clusters in Gram-positive microbes indicates distinct strategies used by bacterial systems to assemble Fe-S clusters.
Keywords: iron sulfur cluster, cysteine desulfurase, SufS, SufU, zinc, sulfurtransferase
Graphical abstract
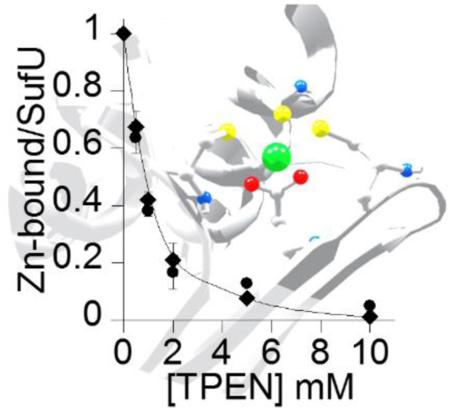
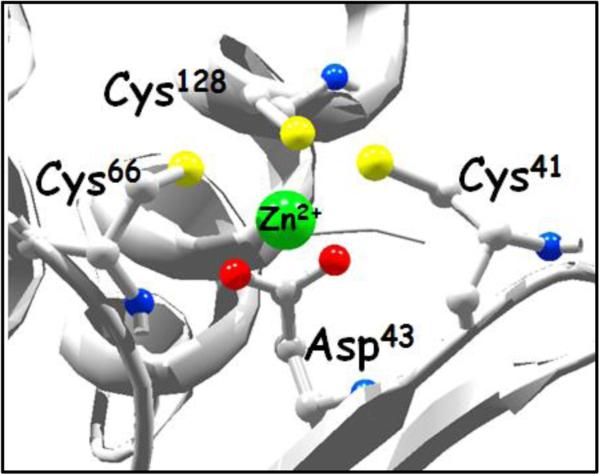
Description. SufU has high affinity for zinc. Inactivation of SufU upon incubation with increasing concentration of TPEN was quantified through its sulfurtransferase activity in SufS assays (◆) and the amount of zinc-bound the protein (●). The calculated Ka is 1017. The background shows the active site of the B. subtilis SufU protein (PDB 2AZH) highlighting the conserved residues, Cys41, Cys66, and Cys128, and Asp43 coordinating the zinc atom.
INTRODUCTION
The study of iron-sulfur (Fe-S) clusters in both prokaryotic and eukaryotic organisms has revealed an expansive catalogue of Fe-S proteins, a wide range of the physiological functions employed by these cofactors, and the complex yet universal machineries required for their biosynthesis. Fe-S proteins participate in several metabolic processes, including enzyme regulation, substrate binding and activation, electron transfer, and regulation of gene expression (1). Simple forms of Fe-S clusters, [2Fe-2S], [3Fe-4S], and [4Fe-4S], can be readily synthesized from sulfide and ferrous/ferric iron under anaerobic conditions in vitro (2). However, due to inherent toxicity of free iron and sulfide, in the cellular environment conditions for the assembly of Fe-S clusters are not as simple and require a group of enzymes dedicated for their assembly and trafficking (3). Three functionality and genetically distinct systems have been identified in bacteria to serve in this capacity: NIF, ISC, and SUF. Their components are found in various combinations within both Gram-negative and Gram-positive bacteria.
One of the first organisms used to study Fe-S cluster biogenesis was the nitrogen-fixing bacteria Azotobacter vinelandii. The initial synthesis of Fe-S units for nitrogenase metalloclusters involves two proteins: NifS – a cysteine desulfurase (4) – and NifU – an Fe-S cluster scaffold (5). The discovery of the function of NifU as a scaffold for Fe-S cluster synthesis was based on its ability to produce transiently-bound [2Fe-2S] and [4Fe-4S] clusters in vitro which, could then be directly transferred to the apo-nitrogenase reductase (6). The proposed functions of these two enzymes, NifS and NifU, established a paradigm that the formation of Fe-S clusters requires a cysteine desulfurase enzyme and a scaffold protein.
Subsequent studies identified the ISC system involved in the housekeeping synthesis of Fe-S clusters which is not restricted to nitrogen fixation. This system also utilizes a cysteine desulfurase and an Fe-S cluster scaffold – IscS and IscU – that are structurally and functionally similar to NifS and to the N-terminal domain of NifU respectively (7). Experimental evidence from resonance Raman, UV/Vis absorption, Mössbauer, and analytical studies showed the formation of [2Fe-2S]2+ clusters on IscU (8-11). Primary sequence similarities are noteworthy between IscU and NifU; both contain three conserved cysteine residues and an invariable aspartate residue located two residues away from the first Cys. Ala-substitution of the Asp39 residue of IscU, known to be conserved among U-type scaffold proteins, resulted in stabilization of Fe-S clusters associated to IscU (8, 12). The crystal structure of IscS-IscUD35A complex from Archeoglobus fulgidus revealed the presence of a 2Fe-2S cluster ligated to three cysteine residues of IscU and the active site cysteine of IscS (12). Based on NifS/IscS and NifU/IscU similarities, the apparent requirement for a cysteine desulfurase and a scaffold protein was suggested to be a universal feature of biological Fe-S cluster formation.
In E. coli, a secondary system for Fe-S cluster formation was identified: the SUF system (13). It was discovered as a backup mechanism to the ISC pathway functional under low iron concentrations and/or oxidative stress (14). In this system, the sulfur mobilization reaction involves the cysteine desulfurase SufS proposed to function in a similar capacity as IscS. In vivo and in vitro studies demonstrated that the activity of this enzyme is dependent on the participation of an intermediate sulfur transfer protein SufE (15). The latter mediates the protected persulfide sulfur transfer reaction from SufS to the proposed scaffold protein SufB when in a complex with SufD and/or SufC (16, 17).
In Gram-positive bacteria, the SUF system is thought to be the sole pathway for the biosynthesis of Fe-S clusters. Gene inactivation studies in Bacillus subtilis (18) and Mycobacterium tuberculosis (19) suggested that the suf genes are essential for survival. Interestingly, the suf operon identified in Gram-positive bacteria does not match those previously studied. While for sulfur mobilization, it also includes the cysteine desulfurase SufS, the subsequent sulfur transfer reaction does not involve a SufE protein, as its coding sequence is absent in B. subtilis and other Gram-positive genomes. On the other hand, the Suf system includes SufB, SufC, and SufD ortholog proteins in addition to SufU, a sulfur acceptor substrate of SufS (20, 21). Because of the sequence similarity to IscU and its ability to enhance the reconstitution of the eukaryotic Fe-S enzyme (Leu1), SufU has been proposed to be an Fe-S cluster scaffold (21).
Nevertheless, functional, structural, and genomic analyses of U-type proteins revealed at least four notable differences between IscU/NifU- and SufU- type proteins: 1) the occurrence of an adjacent gene coding for class II cysteine desulfurase SufS, 2) the presence of an 18-21 amino acid sequence inserted between the second and the third cysteine residues in SufU, 3) a conserved lysine residue occupying the position of the essential histidine preceding the third conserved cysteine, and 4) its ability to enhance the rate of alanine formation of SufS by nearly 200 fold. Alkylation experiments suggest the involvement of a thiol group of SufU during sulfur transfer from SufS persulfide sulfur to a cysteine residue of SufU (20). Subsequent mutagenesis studies showed that all three cysteine residues were mandatory for the SufU sulfurtransferase activity, while only the Cys41 to Ala substitution retained its ability to interact with SufS (21). Interestingly, the structures of B. subtilis and Streptococcus pyogenes SufU showed the presence of a zinc atom coordinated by these three essential cysteine residues along with a conserved aspartate residue (Figure 1) (22, 23). Whether the zinc atom observed in these protein structures is adventitiously bound or an element required for the reactivity of SufU has not been determined. Nevertheless, structures of IscU have also indicated the presence of a zinc atom (24, 25), which has been associated with a defined structured conformation of IscU (25-27).
FIGURE 1.
Active site of SufU. Ribbon representation of B. subtilis SufU protein (PDB 2AZH) showing the conserved residues, Cys41, Cys66, and Cys128, and Asp43 coordinating the zinc atom.
Herein, we demonstrate that SufU is an active participant of the Cys:SufU sulfurtransferase reaction where the ionization state of SufU dictates the second half of the reaction. We found that the zinc atom is tightly bound to SufU and is crucial for its sulfurtransferase activity. Although it has been suggested that SufU functions as an Fe-S cluster scaffold protein in Gram-positive bacteria (21, 28), SufU is not able of constructing Fe-S clusters as previously demonstrated in other U-type proteins in Gram-positive bacteria.
EXPERIMENTAL PROCEDURES
Chemicals
Reagents and chemicals were purchased from Fisher Scientific and Sigma-Aldrich Inc. unless specified. Tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) was purchased from Calbiochem, diethylenetriamine pentetic acid (DTPA) was purchased from MP Biomedicals and Naphthalene-2, 3-dicarboxaldehyde (NDA) was purchased from AnaSpec.
Site directed mutagenesis, expression and purification
The cysteine desulfurase SufS, SufU (pDS63) and the A. vinelandii IscU were purified as described previously (8, 20). The amino acid substitutions were performed by QuickChange Site-Directed Mutagenesis Kit (Stratagene) as specified by the manufacturer. All constructs were made from pDS63, which is the 5′NcoI – 3′XhoI engineered 444 bp fragment of sufU ligated into pET28A(+)(Novagen®) in frame for histidine tag expression at the 3′ end, and SufU expression under lactose control. The variants constructs used in this work were: pDS76 expressing SufUC41A (TGC → GCC), pDS84 expressing SufUC66A (TGT) → GCT), pDS77 expressing SufUC128A (TGT) → GCT), pDS85 expressing SufUD43A (GAC) → GCC). The correct codon substitution was determined by DNA sequencing (Genewiz®) The expression of SufU variants was accomplished by transforming each plasmid into CaCl2 chemically competent E. coli cells; pDS76 and pDS77 were transformed into E. coli C41 (DE3), while pDS84 and pDS85 were transformed into E. coli Rosetta (DE3). Transformed cells were selected on LB agar plates containing kanamycin 40 μg.μL−1. Single colonies were used to inoculate 500 mL of LB medium with the same antibiotic concentration as the solid medium and outgrown at 300 RPM/37 °C until OD600 of 0.5 was reached, at this point cells were induced with L-lactose (0.2%) and further grown at 25°C overnight (16 h). The cells were then harvested by centrifugation at 6000 g for 10 min and stored at −20°C.
The cell pellets were re-suspended in 25 mM Tris-HCl pH 8, 0.3 M NaCl, 10% glycerol (buffer A) and disrupted by EmulsiFlex-C5 high pressure homogenizer (Avestin), followed by centrifugation at 12,800 g for 20 min to remove the cell debris. The supernatant was loaded onto a FPLC IMAC-Ni2+ (GE Healthcare) column and washed with 5 column volumes of buffer A. The bound proteins were eluted through a step gradient (4% and 30%) of 25 mM Tris-HCl pH 8, 0.3 M NaCl, 10% glycerol, 500 mM imidazole (buffer B). SufU variants were displaced from the column at 30% buffer B. Fractions containing each SufU were pooled, diluted 5-fold, and then loaded onto a FPLC Q-sepharose fast flow column (GE Healthcare) pre equilibrated with 25 mM Tris-HCl pH 8, 10% glycerol (buffer C). The column was washed with 5 column volumes of buffer C and the bound sample was eluted through a linear gradient (0-70%) of 20 column volumes of 25 mM Tris-HCl pH 8, 1 M NaCl, 10% glycerol (buffer D). SufU variants were displaced from the column when the concentration of buffer D reached 45%. Fractions containing purified SufU were pooled, frozen in liquid nitrogen and stored at −80 °C. Elution profiles were followed at 280 nm. All of the protein purifications were monitored by SDS-PAGE, and the protein concentrations were determined by the method of Bradford et al (29), using BioRad protein assay kit and bovine serum albumin as a standard.
Cysteine desulfurase activity
Cysteine desulfurase activity was determined by quantifying the amount of both products, alanine by derivatization with NDA (20) and sulfide by the formation of methylene blue (30). Unless indicated, the reactions (800 μl) contained 1.38 μM SufS, 6.9 μM SufU, 0.5 mM cysteine in 50 mM MOPS (pH 8) buffer containing 2 mM dithiothreitol (DTT).
Apo-SufU and reconstitution
Purified SufUWT was incubated with 100 mM diethylenetriamine pentetic acid for 2 h at room temperature, followed by 3 dialysis cycles, each against 2 L of 25 mM Tris-HCl pH 8, 10% glycerol for 2 hrs. After dialysis, the sample was loaded onto a Q-Sepharose column. Sample was washed with 5 column volumes of 25 mM Tris-HCl pH 8, 10% glycerol and eluted with 0.6 M NaCl in the same buffer. Combined fractions were frozen in liquid nitrogen and stored at −80°C. On-column SufU-Zn reconstitution was conducted by charging an IMAC column with a 150 mM ZnCl2 solution and equilibrating it with 25 mM Tris-HCl pH 8. His-tagged Apo-SufU was loaded onto the column and washed with equilibration buffer. The protein was eluted with 25 mM Tris-HCl pH 8, 0.6 M imidazole. Combined fractions containing reconstituted SufU were dialyzed against 2 L of 25 mM Tris-HCl pH 8 (2X). Both ICP-OES and cysteine desulfurase activity assays were carried out.
The divalent metal reconstitutions were conducted as follows: A stock solution in the presence of 5 mM EDTA in a 1:1 ratio to each metal was used. Apo-SufU (0.1 mM) was incubated with a solution of 0.5 mM of each respective metal containing 0.5 mM EDTA for 2 hrs. The activity of the reconstituted SufU was determined by cysteine desulfurase assays containing 0.5 mM cysteine, 0.01 mg of SufS, 2 mM DTT, and sub-saturating concentrations of reconstituted SufU (1:5 molar ratio of SufS:SufU). The percent relative activity was normalized to the rate of sulfide formation when as-isolated SufU was assayed under the same conditions (180 ± 15 nmol sulfide.min−1.mg−1). All reactions were carried out in the presence of 50 mM Mops pH 8 at room temperature.
Inductively coupled plasma optical emission spectrometry (ICP-OES)
Analysis of the zinc content within the protein was performed by ICP-OES. A standard curve for zinc was constructed using varying concentrations of ZnCl2 (0, 0.5, 1, 2.5 and 5 ppm) in the presence of 25 mM Tris-HCl pH 8, and 2% HNO3 in a final volume of 10 mL. Each respective sample was brought up to a final volume of 10 mL with HNO3, the final concentration of acid in the samples were also 2%. Samples were centrifuged at 5000 rpm for 20 min prior to analysis. The amount of zinc in each sample was calculated through a linear regression.
Iron-Sulfur Cluster assembly
A. vinelandii IscU, B. subtilis SufU, apo-SufU and SufUD43A cluster assembly reactions were carried out in an anaerobic chamber (Coy) equilibrated with 5% H2 balanced with N2 gas. Unless stated, each reaction contained 0.4 μM IscS or SufS; 14 μM IscU, SufU or apo-SufU; 42 μM Fe, 42 μM Cys and 42 μM DTT. Samples that required further purification for isolation of an Fe-S cluster loaded protein were passed onto an IMAC-Ni2+ column previously equilibrated with 25 mM Tris-HCl pH 8, 0.3 M NaCl and eluted with 25 mM Tris-HCl pH 8, 0.3 M NaCl, 150 mM imidazole.
UV-Vis absorption and Circular Dichroism spectroscopy
Secondary structure was determined by circular dichroism (CD) using an AVIV Circular Dichroism spectrometer. All protein samples were at 10 μM in 10 mM sodium phosphate Buffer pH 7. Scans were performed from 200 to 250 nm range with 1-nm increments. Each resulting spectrum was generated from the average of 10 scans. The visible CD spectra and UV/Vis absorption spectra were determined for samples subjected to Fe-S cluster assembly. Each visible CD scan (300 – 700 nm) was obtained with a 5 mm-pathlength cuvette and an 1-nm bandwidth. The final spectrum was generated from the average of 10 scans. The UV/Vis absorption spectra were determined in a Cary 50 spectrophotometer from 250-600 nm range with 1-nm increments.
TPEN titration
A 50 mM TPEN solution was prepared in ethanol and subsequently diluted in 50 mM Mops pH 8. SufUWT (135 μM) was incubated for 2 hrs with different concentrations of TPEN (0.5, 1, 2.5, 5 mM). After incubation samples were dialyzed twice, each dialysis against 2 L Tris-HCl pH 8. ICP-OES and SufS cysteine desulfurase assays, as described above, were carried out to determine the amount of zinc bound to the protein. The reaction with TPEN is assumed to reach equilibrium (equation 1), where the concentration of free zinc was considered to be negligible. Using the reported affinity constant of TPEN for zinc (Ka TPEN of 1016 M−1), the equation 2 was used to calculate the binding affinity of SufU for zinc.
| (1) |
| (2) |
The concentration of SufUZn was calculated from the Cys:SufU sulfurtransferase assay, when using sub-saturating concentrations of SufU. At equilibrium, the concentration of apo-SufU (SufUapo) was equal to the concentration of SufU added to the reaction minus SufUZn, and the concentration of TPENZn was presumed to be the same as SufUapo.
Zinc-EXAFS
Reactions were carried out anaerobically in the presence of 28 μM SufS, 0.84 mM SufU, 4.2 mM L-cysteine and 4.2 mM ferrous ammonium sulfate, and 4.2 mM DTT. Zn X-ray absorption spectra were measured at Stanford Synchrotron Radiation Lightsource (SSRL) beamline 7-3 using their in-house EXAFS equipment. Samples were frozen in custom-made Lucite cuvettes (20 × 3 × 2 mm) and mounted inside an Oxford instruments CF1208 liquid helium cryostat cooled to 10K. Fluorescent X-rays were measured using a 27-element Ge detector from Canberra Industries, equipped with Cu filters and Soller slits to minimize scattered radiation. Samples were prepared with 20% glycerol to minimize ice crystal formation. Data analysis and curve fitting were performed using the EXAFSPAK suite of programs (31) with EXAFS phase and amplitude functions calculated using the FEFF 7.0 single scattering interface (32).
RESULTS
pH-activity profile of SufS reaction
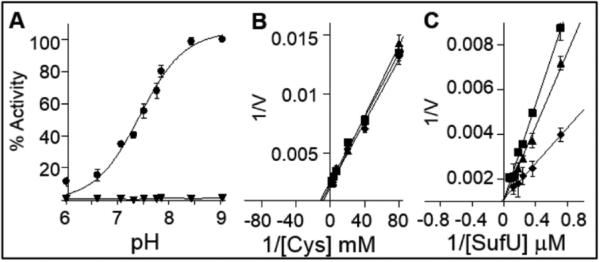
In B. subtilis, the essential cysteine desulfurase SufS catalyzes the Cys:SufU sulfurtransferase reaction. Our previous studies showed that the first half of this reaction includes the formation of an enzyme S-covalent intermediate at Cys364 residue of SufS followed by the release of the first product alanine. The second half of the reaction involves a persulfide sulfur transfer step to a thiol on the sulfur acceptor SufU protein (20). Two possible sulfur transfer mechanisms could involve the second half of this reaction: 1) SufU could act as an electrophile, and sulfur transfer could involve the nucleophilic attack of the enzyme's terminal persulfide onto a thiol of the acceptor molecule, or 2) SufU could act as a nucleophile leading the nucleophilic attack onto the persulfide sulfur. In order to gain insight into the chemical steps dictating the second half of this reaction, we inspected the pH-activity profile of the SufS reaction in the presence and in the absence of SufU (Figure 2A). The reaction rate in the presence of the sulfur acceptor protein showed the occurrence of at least one ionization event with an associated pKa of 7.34, similar to the profile reported for the E. coli SufS in the presence of the sulfur acceptor SufE (17). The pH-dependency of the SufS reaction in the presence of SufU suggested that the deprotonated form of SufU could be the active form of the substrate dictating the second half of the reaction. Therefore, we hypothesized that, at pH values lower than the calculated pKa, the rate of the reaction would be limited by the concentration of the catalytic competent form of SufU (i.e. deprotonated SufU). Based on this proposal, the pH of the reaction would affect the apparent affinity of the enzyme for SufU, but not its turnover rate (Kcat). Supporting this model, SufU saturation curves showed the total concentration of SufU required to reach half of maximum reaction rate (KM app) varied with pH (i.e. at lower pH values the relative concentration of deprotonated SufU is lower) (Figure 2C). Moreover, under SufU-saturating concentrations, the pH of the reaction had no effect on the Cys saturation curves (Figure 2B), suggesting that the first half of the reaction was not affected by this ionization event and that the protonated form of the substrate was not an inhibitor of the reaction. These results support a reaction mechanism in which the second half of the reaction is dependent on the nucleophilic attack of a deprotonated thiol group of SufU on SufS’ persulfide thiol intermediate.
FIGURE 2.
pH-activity profile of SufS reaction. A) The pH dependency of SufS reaction in the absence (▼) and in the presence of SufU (●). The data was fitted to the Henderson-Hasselbalch equation, , where Act is the relative activity (%) at each pH value in relation to the maximum activity determined at pH 8. The pKa of this ionization event was calculated to be 7.34. B) Double reciprocal plots of alanine formation under steady state conditions of cysteine substrate saturation curve. Reactions were carried out in the presence of 1.3 μM SufS, 39 μM SufU and variable concentrations of L-cysteine (0.0125 – 0.5 mM). C) Double reciprocal plots of alanine formation under steady state condition of SufU substrate saturation curve. Reactions were carried out in the presence of 1.3 μM SufS 0.5 mM L-cysteine, and variable concentration of SufU (1.3 – 13 μM). All reactions were performed in panel B and C were at pH 7.4 (■), pH 7.7 (▲) and pH 8.1 (◆).
SufU is a zinc-dependent sulfurtransferase
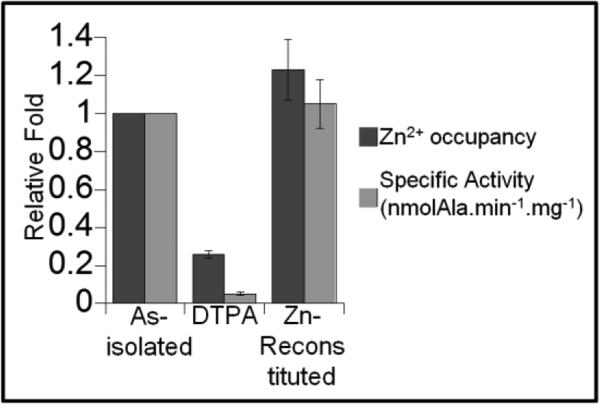
The active role of SufU in the second half of the SufS sulfurtransferase reaction led to the investigation of structural and functional features associated with this activity. Although the lack of activity observed for Cys to Ala SufU variants could be explained by the elimination of functional thiol groups participating in sulfurtransfer reactions (Figure S1, (33)), it also could be a consequence of structural changes resulting from elimination of residues coordinating the zinc. Far-UV circular dichroism spectra of Cys to Ala SufU variants along with Asp43 to Ala substitution showed distinct spectral features associated with alpha-helical and beta-sheet content when compared to the wild-type protein (Figure S2A). In addition, ICP-OES analysis shows that the zinc content associated with SufU was determined to be 1.1±0.2 zinc/monomer, whereas SufU variants displayed 60-85% reduction in the zinc content (Figure 4 and S1).
FIGURE 4.
Zinc-dependent sulfurtransferase activity of SufU. The cysteine desulfurase activity was measured by the formation of alanine through HPLC and normalized to the activity of wild-type shown in grey bars. The assays contained 1.3 μM of SufS and 6.9 μM of as-isolated SufU, apo-SufU (DTPA), or on-column reconstituted SufU. The relative zinc+ content measured through ICP-OES is shown in dark grey bars.
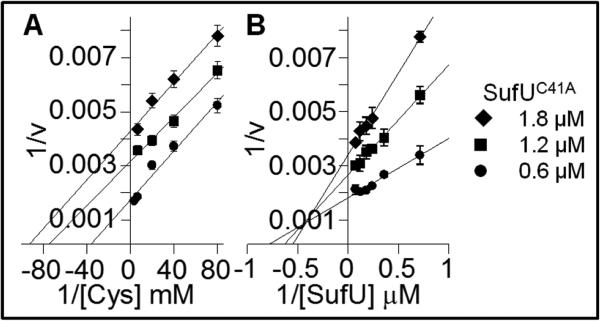
Whereas SufU variants showed compromised zinc-binding which impacted both its structure and sulfurtransferase function, two particular substitutions retained its ability to interact with SufS although not in a productive manner. First, when co-expressed, SufUD43A is isolated in a complex with SufS, similar to the observed IscS and IscUD39A complex (8), but devoid of Fe-S clusters (data not shown). Second, SufUC41A interacts with SufS inhibiting SufU participation in the reaction, but not through a competitive mode as initially proposed (33). Instead, SufUC41A was still able to interact with SufS acting as a non-competitive inhibitor of SufU wild-type and uncompetitive inhibitor of cysteine (Figure 3). Interestingly, previously reported binding experiments demonstrating complex formation between SufS and SufUC41A only in the presence of cysteine (33) are in agreement with the inhibition mode of SufC41A described here.
FIGURE 3.
SufUC41A inhibition of Cys:SufU sulfurtransferase reaction of SufS. Double reciprocal plots of alanine formation under steady state conditions are displayed for cysteine (A) and SufU (B) substrate saturation curves in the presence of 0.6 μM (●), 1.2 μM (■) and 1.8 μM (◆) the concentration of SufUC41A. A) Reactions were carried out in the presence of 1.3 μM SufS, 13 μM SufUWT and variable concentrations of cysteine (0.0125 – 0.5 mM). B) Reactions were carried out in the presence of 1.3 μM SufS, 0.5 mM cysteine, and variable concentrations of SufUWT (1.3 – 13 μM).
Because of the correlation between the lack of activity and decreased zinc content in SufU variants, we hypothesized that the zinc atom could be an important element for the structural integrity of the SufU protein, maintaining the Cys residues in the proper conformation and in its reduced state. In addition, zinc is known to increase the nucleophilicity of its ligands functioning as a Lewis acid, thus potentially enabling SufU's participation on the second half of the reaction. Therefore, we sought to determine if the apo-form of SufU could be a catalytically competent substrate. As-expressed, as-isolated both A. vinelandii IscU and B. subtilis SufU contain approximately stoichiometric levels of zinc, however unlike A. vinelandii IscU, the zinc atom associated with SufU could not be removed upon 100 mM EDTA treatment. Incubation with the stronger chelator DTPA, however, efficiently removed the metal ion. The apo-SufU showed a distinct Far-UV CD spectrum (Figure S2B) and was not active in the sulfurtransferase assay (Figure 4). The zinc-dependent activity of SufU was, then, determined by the ability of this protein to fully regain activity upon reconstitution. Direct incubation of SufU with a ZnCl2 solution resulted in protein precipitation. Nevertheless, effective activation was accomplished by two different approaches. First, complete recovery of SufU activity was achieved by passing the apo-protein through a zinc-charged IMAC column (Figure 4). Alternatively, reactivation was also achieved by incubating apo-SufU with 10 mM Zn-EDTA solution, where the reconstituted protein recovered 70% of the activity observed for the as-isolated protein (Figure S3).
SufU binds zinc with high affinity
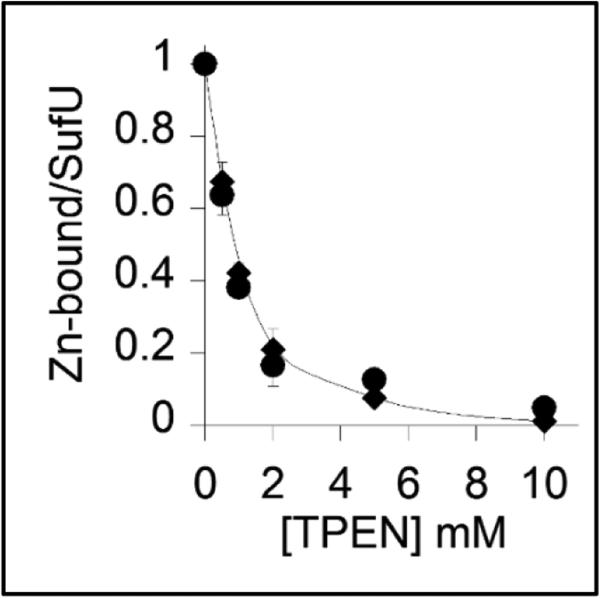
Metal reconstitution experiments showed zinc-dependent SufU activity. However, the presence of this metal in LB medium is estimated to be approximately 10 μM (34), which could lead to adventitious metal misincorporation. To address this concern, the binding affinity of SufU for zinc was determined in a titration experiment with the zinc chelator TPEN as described by Collet and Jakob (35, 36) (Figure 5). As-isolated SufU was incubated with various concentrations of TPEN followed by dialysis. The residual sulfurtransferase activity (Figure 5, diamonds) overlays well with the quantification of the remaining zinc associated to SufU after dialysis (Figure 5, circles). Prolonged incubation times of the protein with TPEN did not change the inhibition pattern (data not shown). Using the known binding affinity of TPEN for zinc (1016 M−1) and the zinc-dependent activity profile of SufU, the Ka of SufU for zinc was calculated to be 1017 M−1. This value is among the highest affinity constants ever reported for zinc-dependent enzymes. Moreover, metal reconstitution with other divalent metals did not show recovery of SufU activity (Figure S3), indicating that the binding of SufU to other metals is either not tight and/or does not recover its active role in participating in the second half of the SufS sulfurtransferase reaction.
FIGURE 5.
Zinc binding affinity to SufU. SufU (135 μM) was incubated with increasing concentrations of TPEN for 3 hours followed by dialysis. The amount of zinc-bound SufU was determined by ICP-OES (●) and by the SufS sulfurtransferase assay using 0.22 μM SufS and 0.5 mM cysteine (◆).
SufU does not act as a standard Fe-S cluster assembly scaffold
Prior suggestions that SufU serves as a platform for the assembly and delivery of Fe-S clusters were based on its amino acid sequence similarity to IscU (28), and the ability of B. subtilis SufU to enhance rates of activation of the human [4Fe-4S] cluster Leu1 (21) and Thermatoga maritima SufU to activate a [2Fe-2S] cluster ferredoxin (37, 38). Additional support for this proposal was obtained by isolation of an Fe-S cluster bound form of SufU variant carrying an Ala substitution for the Asp43 residue (21, 38). In agreement with previous reports (21, 38), recombinant expression of the B. subtilis SufUD43A variant in our hands also resulted in accumulation of SufU having a low level Fe-S cluster occupancy after protein isolation (data not shown). In vitro Fe-S cluster assembly followed by purification of SufUD43A showed the presence of Fe-S species associated with this variant protein (Figure S4).
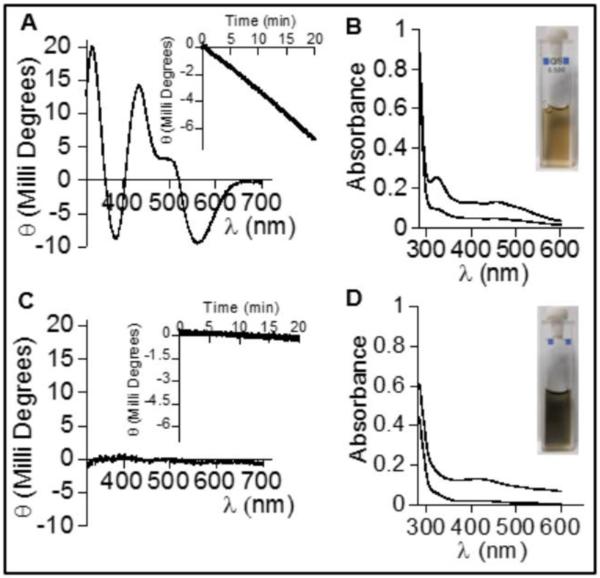
In contrast, repeated attempts to isolate and characterize Fe-S species associated with wild-type apo or zinc-bound SufU proteins have been unsuccessful (21, 38). Similarly, Albrecht and collaborators were unable to characterize Fe-S clusters that were proposed to be associated with SufU by using electron paramagnetic resonance (EPR) and Mossbauer (21). Our interpretation of these results is that the Fe-S clusters proposed to be associated with SufU could have actually represented adventitiously bound iron-sulfide species. Cowan and collaborators have also reported on their unsuccessful attempts to reconstitute wild-type Thermatoga maritima “IscU” protein, which exhibits a primary structure that is more closely aligned with the Gram-positive SufU family than with the canonical IscU family (38). In contrast, experimental conditions to promote catalytic Fe-S cluster assembly and detection on wild-type IscU have been well-characterized (9, 10). Using similar conditions previously described to assemble Fe-S species on IscU, we were able to reproduce cluster-bound IscU species having nearly identical UV/Vis absorption and Vis CD spectra as previously reported (Figure 6A and B). In contrast, when using these same conditions, SufU protein did not generate comparable spectra (Figure 6C and D). Namely, the UV/Vis absorption spectra after 30 min incubation with Fe, Cys, DTT, and SufS showed features that resembled the accumulation of Fe-sulfide species having a characteristic blackish color (Figure 6D, inset). Also, the Vis CD absorption spectrum remained silent throughout the course of attempted cluster assembly (Figure 6C). Finally, purification of SufU following the two-hour incubation period yielded a colorless solution with no evidence of a bound Fe-S species as evidenced by UV/Vis absorption or Vis-CD spectra (Figure S6). Interestingly, after purification this protein still retained its sulfur-transferase activity to levels relative to its zinc content (Figure S6, inset). Fe-S cluster assembly reactions containing the apo-form of SufU incubated with iron prior to the addition of SufS and cysteine showed no visible change up to two hours of incubation, while reconstitution experiments using excess of iron and sulfide as the sulfur source resulted in the appearance of blackish-colored solution and silent Vis-CD spectrum (data not shown). Purification of SufU after cluster assembly by anion-exchange, gel filtration, or Ni-IMAC column resulted in no Fe-S cluster species associated with the protein, as indicated by iron analysis, UV-Vis absorption and CD spectra (Figure S5).
FIGURE 6.
Fe-S cluster assembly on IscU and SufU. Reactions were performed as described in the materials and methods. The Vis CD spectrum after 20 min incubation shows the formation of the [2Fe-2S] cluster as previously reported for IscU (A) and distinct from the CD spectrum observed for SufU (C). The insets on panels A and C show the kinetics of cluster formation by monitoring the intensity of the peak at 560 nm. The UV/Vis absorption spectrum at 5 min and after 3 hours incubation during Fe-S cluster assembly conditions for IscU (B) and SufU (D). The inset panels B and D show the color of the sample at the end of the experiment.
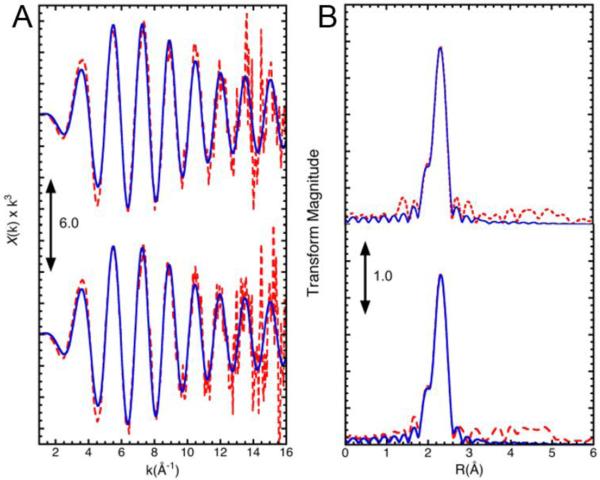
In addition, we have inspected the zinc coordination of SufU samples as-isolated and when subjected to Fe-S cluster assembly conditions (i.e. in the presence of SufS, cysteine, Fe2+, and DTT under anaerobic conditions). In this case, Extended X-Ray Absorption Fine Structure (EXAFS) was used to probe the zinc coordination of SufU during Fe-S cluster assembly conditions. If SufU could transiently coordinate Fe-S clusters, the zinc coordination would be perturbed since the proposed site of cluster assembly share the residues identified as ligands of the zinc. In agreement with results from UV/Vis and CD experiments (Figure 6), EXAFS shows that the zinc coordination remains unaltered during cluster assembly conditions, and does not provide evidence for assembly of Fe-S clusters onto SufU. In both samples, the phase shifted Fourier transform of the Zn K-edge spectrum showed a large peak at 2.3 Å accompanied by a minor peak at 2 Å (Figure 7B, red trace). The k3 weighted EXAFS data of SufU (Figure 7A, red trace) fits well with a model in which the zinc is coordinated by 3 S atoms at 2.3 Å and 1 O atom at 2.0 Å (Figure 7B, blue trace) using parameters described in Table I. This model matches well with the reported ligands and associated distances for the zinc coordination on the B. subtilis SufU NMR structure (Figure 1, (22)). Nevertheless, results from EXAFS analysis provides further evidence that under conditions observed for the assembly of Fe-S cluster on other scaffolds, SufU retains the zinc atom and does not coordinate transient Fe-S cluster species.
FIGURE 7.
EXAFS spectra (A) and Fourier transforms (B) of SufU of 0.84 mM as-isolated SufU (top) and SufU under Fe-S cluster assembly conditions (bottom). Cluster assembly experiments were conducted as described in the materials and methods. For each panel: (red dashed) spectrum; (blue solid) simulation. The simulated fits use the parameters in Table 1.
Table I.
EXAFS Curving-Fitting Parameters*
| Sample | Interaction | N | R (Å) | σ2 (Å2) | Δ E0 | F |
|---|---|---|---|---|---|---|
| Resting | Zn-S | 3 | 2.321 (0.003) | 0.0032 (0.0001) | −15.5 (0.8) | 0.375 |
| Zn-O | 1 | 2.053 (0.007) | 0.0017 (0.0005) | |||
| Assembly Conditions | Zn-S | 3 | 2.324 (0.004) | 0.0034 (0.0002) | −15.1 (1.0) | 0.618 |
| Zn-O | 1 | 2.048 (0.010) | 0.0022 (0.0007) | |||
Fits used in Figure 6 when N = number of backscattering atoms used in EXAFS fit; R = distance used in EXAFS fit; σ2 = mean-squared deviation (Debye–Waller factor) used in fit; σ = equivalent root-mean-squared deviation (Debye–Waller factor); ΔE0 = offset in E0; F = EXAFS fit quality = √[Σ(χo – χc)2k6/Σχo2k6] where χo = observed EXAFS; χc = calculated EXAFS. Figures in parentheses are the standard deviations for each fitted parameter. Values in plain text were left to float for calculations, while values in italics were held fixed.
DISCUSSION
One of the first steps of Fe-S cluster biogenesis in B. subtilis involves the mobilization of sulfur from cysteine by the cysteine desulfurase SufS. In a previous report (20), we have described a double displacement mechanism of the Cys:SufU sulfurtransferase reaction where in the enzyme shows high affinity for the second substrate SufU (KM SufU = 3 μM). Here we show that SufU is an active participant in the second half of this reaction. Results from pH-activity profile suggest that the deprotonated form of SufU promotes the nucleophilic attack onto the terminal persulfide thiol enzyme intermediate, thus controlling the overall reaction rate. Therefore, it is likely that under physiological conditions, the sulfur transfer reaction from the SufS intermediate to the acceptor SufU is the rate limiting step.
The involvement of a thiol residue participating in persulfide sulfur transfer led to the investigation of the identity of the cysteine residue(s) participating in this reaction step. However, the interdependency of cysteine residues involved in the structure and function of this protein did not allow a straightforward investigation using site-directed mutagenesis. The structure of the B. subtilis SufU (22) showed the presence of a zinc atom displaying a tetra-coordination by four conserved residues (Cys41, Cys66, Cys128, and Asp43) (Figure 1). Individual Ala-substitutions of these residues affected three mutually dependent aspects of SufU structure and function: (i) disrupted zinc binding, (ii) impacted secondary structure, and (iii) eliminated sulfurtransferase activity. Moreover, the zinc-dependent activity profile was also observed in wild-type SufU. The apo-form of SufU also displayed altered secondary structure and loss of its capacity to serve as a substrate of SufS. Most importantly, the zinc-dependent sulfurtransferase profile of SufU was further supported by complete recovery of SufU's activity upon zinc reconstitution.
Nonetheless, since we have not been able to identify an experimental condition that impaired SufU's function without disturbing zinc binding and/or protein structure, the precise role of zinc as a structural and/or catalytic element remains to be uncovered. The zinc may stabilize the protein structure in the active conformation while preventing intramolecular disulfide formation. Alternatively, the zinc may act as a Lewis acid by lowering the pKa of the thiol making it a better nucleophile during the sulfurtransfer step or directly accepting a partial coordination for the incoming sulfur before subsequent transfer to a final acceptor protein. Nevertheless, a mechanistic role for zinc in Fe-S cluster biogenesis was not previously proposed and the zinc-dependent sulfurtransferase activity of SufU reported here indicates a new role for this metal in biology.
As a matter of fact, the binding affinity of SufU for zinc is high (Ka = 1017 M−1) which is amongst the highest binding constants reported so far for zinc-dependent enzymes (35, 36, 39). Based on spectroscopic and structural characterization of Fe-S clusters bound to IscU (9-12), the candidate ligands for SufU would be the same residues coordinating the zinc (Cys41, Cys66, Cys128, and Asp43). In addition, SufU sequences also contain a conserved lysine residue in place of the conserved histidine residue (His 105 of IscU) implicated in cluster binding (40) or stabilization (12). Furthermore, the calculated KdSufU for zinc of a fentomolar scale indicates a very tight binding of the metal to the protein making it unlikely that the zinc dissociates under conditions of Fe-S cluster assembly.
The same approach taken to establish the role of IscU as an Fe-S cluster scaffold was employed here to investigate initial proposals suggesting SufU involvement as an Fe-S cluster scaffold protein (3, 21, 28). While the binding of zinc to IscU did not prevent its ability to coordinate Fe-S clusters, SufU failed to coordinate transient Fe-S cluster species and retained its zinc ligand (Figure 6). Moreover, assembly reaction conditions previously reported also failed to yield a cluster-bound SufU (21, 38). Fe-S cluster assembly experiments using the apo-form of SufU also resulted in no detection of a Fe-S cluster-bound species (Figure S5). In agreement with previous reports (21, 38), substitution of the zinc-ligand aspartate 43 residue by alanine (SufUD43A) affects zinc binding and enables the assembly of Fe-S clusters. Substitution of Asp39 of IscU abolishes its in vivo function (41). In B. subtilis, substitution of this strictly conserved residue eliminated the ability of SufU to participate in the sulfurtransferase reaction of SufS. Thus, in vitro Fe-S reconstitution of the inactive variant SufUD43A led to proposals involving the role of this protein as a standard Fe-S cluster scaffold. In contrast, the results presented here, while provided additional confirmation of prior studies reporting unsuccessful attempts to isolate cluster-bound forms of wild-type SufU (21, 38), offered experimental evidence for the requirement of zinc for SufU's role as an intermediate in sulfur mobilization.
The B. subtilis Suf system includes the cysteine desufurase SufS and the sulfur acceptor zinc-dependent sulfurtransferase SufU. Both are involved in the sulfur mobilization reaction for the biosynthesis of Fe-S clusters. In the more extensively studied E. coli Suf system, sulfur mobilization involves the SufS cysteine desulfurase and the sulfur acceptor SufE. Together SufS and SufE mediate protected sulfur transfer reactions from cysteine to the proposed Fe-S cluster scaffold SufB (17). While the identity of the physiological sulfur acceptor(s) of the B. subtilis SufU have not yet been established, the suf operon also encodes SufB, SufC, and SufD proteins indicating that one or more of these proteins are likely candidates to serve this function. This possibility is suggested based on analogy of the Fe-S cluster biogenesis by the E. coli Suf system, where SufB in B. subtilis is a potential site for the assembly of Fe-S clusters. Although the amino acid sequence of IscU and SufU proteins are less than 20% identical to that of SufE, their structures display similar folding.(42) Based on these observations, we suggest that SufU could represent an evolutionary intermediate of two distinct types of cysteine desulfurases partners. Mainly, SufU retains phylogenetic proximity to standard Fe-S cluster scaffold IscU proteins while displaying a function analogous to exclusive sulfurtransferase activity similar to SufE.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank D.R. Dean for critical reading of the manuscript. This work was supported by National Science Foundation (MCB-1054623) to PDS.
ABBREVIATIONS
- Fe-S cluster
iron sulfur cluster
- DTT
dithiotreitol
- TPEN
tetrakis-(2-pyridylmethyl)ethylenediamine
- NDA
naphthalene-2, 3-dicarboxaldehyde
- ICP-OES
Inductively coupled plasma optical emission spectrometry
- DTPA
diethylenetriamine pentetic acid
- CD
circular dichroism
- EXAFS
extended x-ray absorption fine structure
Footnotes
SUPPORTING INFORMATION AVAILABLE
Alanine substitution of zinc ligands causes an effect on the sulfurtransferase activity, zinc content (Figure S1), and secondary structure (Figure S2). The binding of zinc to SufU is specific and other divalent metals cannot restore its sulfurtransferase activity (Figure S3). Purified of SufU43DA after reaction under Fe-S cluster assembly conditions shows the UV-Vis absorption and CD spectra characteristic of Fe-S clusters associated to the protein (Figure S4). On the other hand, SufU (apo-form or zinc-bound) after Fe-S cluster assembly and purification show silent Vis-CD spectrum, and displays zinc content comparable to its sulfurtransferase activity (Figure S5 and Figure S6). Supplemental materials may be accessed free of charge online at http://pubs.acs.org.
REFERENCES
- 1.Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- 2.Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature's modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- 3.Roche B, Aussel L, Ezraty B, Mandin P, Py B, Barras F. Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim Biophys Acta. 2013;1827:455–469. doi: 10.1016/j.bbabio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Zheng L, White RH, Cash VL, Jack RF, Dean DR. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc Natl Acad Sci U S A. 1993;90:2754–2758. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuvaniyama P, Agar JN, Cash VL, Johnson MK, Dean DR. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc Natl Acad Sci U S A. 2000;97:599–604. doi: 10.1073/pnas.97.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos PC, Smith AD, Frazzon J, Cash VL, Johnson MK, Dean DR. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J Biol Chem. 2004;279:19705–19711. doi: 10.1074/jbc.M400278200. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 8.Raulfs EC, O'Carroll IP, Dos Santos PC, Unciuleac MC, Dean DR. In vivo iron-sulfur cluster formation. Proc Natl Acad Sci U S A. 2008;105:8591–8596. doi: 10.1073/pnas.0803173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandramouli K, Unciuleac MC, Naik S, Dean DR, Huynh BH, Johnson MK. Formation and properties of [4Fe-4S] clusters on the IscU scaffold protein. Biochemistry. 2007;46:6804–6811. doi: 10.1021/bi6026659. [DOI] [PubMed] [Google Scholar]
- 10.Urbina HD, Silberg JJ, Hoff KG, Vickery LE. Transfer of Sulfur from IscS to IscU during Fe/S Cluster Assembly. J Biol Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- 11.Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- 12.Marinoni EN, de Oliveira JS, Nicolet Y, Raulfs EC, Amara P, Dean DR, Fontecilla-Camps JC. (IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew Chem Int Ed Engl. 2012;51:5439–5442. doi: 10.1002/anie.201201708. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 14.Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 15.Outten FW, Wood MJ, Munoz FM, Storz G. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. 2003;278:45713–45719. doi: 10.1074/jbc.M308004200. [DOI] [PubMed] [Google Scholar]
- 16.Layer G, Gaddam SA, Ayala-Castro CN, Ollagnier-de Choudens S, Lascoux D, Fontecave M, Outten FW. SufE Transfers Sulfur from SufS to SufB for Iron-Sulfur Cluster Assembly. J Biol Chem. 2007;282:13342–13350. doi: 10.1074/jbc.M608555200. [DOI] [PubMed] [Google Scholar]
- 17.Selbach BP, Pradhan PK, Dos Santos PC. Protected Sulfur Transfer Reactions by the Escherichia coli Suf System. Biochemistry. 2013;52:4089–4096. doi: 10.1021/bi4001479. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Ehrlich SD, Albertini A. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huet G, Daffe M, Saves I. Identification of the Mycobacterium tuberculosis SUF machinery as the exclusive mycobacterial system of [Fe-S] cluster assembly: evidence for its implication in the pathogen's survival. J Bacteriol. 2005;187:6137–6146. doi: 10.1128/JB.187.17.6137-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selbach B, Earles E, Dos Santos PC. Kinetic analysis of the bisubstrate cysteine desulfurase SufS from Bacillus subtilis. Biochemistry. 2010;49:8794–8802. doi: 10.1021/bi101358k. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht AG, Netz DJ, Miethke M, Pierik AJ, Burghaus O, Peuckert F, Lill R, Marahiel MA. SufU is an essential iron-sulfur cluster scaffold protein in Bacillus subtilis. J Bacteriol. 2010;192:1643–1651. doi: 10.1128/JB.01536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornhaber GJ, Snyder D, Moseley HN, Montelione GT. Identification of zinc-ligated cysteine residues based on 13Calpha and 13Cbeta chemical shift data. J Biomol NMR. 2006;34:259–269. doi: 10.1007/s10858-006-0027-5. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Oganesyan N, Shin DH, Jancarik J, Yokota H, Kim R, Kim SH. Structural characterization of an iron-sulfur cluster assembly protein IscU in a zinc-bound form. Proteins. 2005;59:875–881. doi: 10.1002/prot.20421. [DOI] [PubMed] [Google Scholar]
- 24.Ramelot TA, Cort JR, Goldsmith-Fischman S, Kornhaber GJ, Xiao R, Shastry R, Acton TB, Honig B, Montelione GT, Kennedy MA. Solution NMR structure of the iron-sulfur cluster assembly protein U (IscU) with zinc bound at the active site. J Mol Biol. 2004;344:567–583. doi: 10.1016/j.jmb.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Tonelli M, Markley JL. Disordered form of the scaffold protein IscU is the substrate for iron-sulfur cluster assembly on cysteine desulfurase. Proc Natl Acad Sci U S A. 2012;109:454–459. doi: 10.1073/pnas.1114372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prischi F, Pastore C, Carroni M, Iannuzzi C, Adinolfi S, Temussi P, Pastore A. Of the vulnerability of orphan complex proteins: the case study of the E. coli IscU and IscS proteins. Protein expression and purification. 2010;73:161–166. doi: 10.1016/j.pep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Markley JL, Kim JH, Dai Z, Bothe JR, Cai K, Frederick RO, Tonelli M. Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery. FEBS Lett. 2013;587:1172–1179. doi: 10.1016/j.febslet.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen JS, Mortenson LE. Inhibition of methylene blue formation during determination of the acid-labile sulfide of iron-sulfur protein samples containing dithionite. Anal Biochem. 1977;79:157–165. doi: 10.1016/0003-2697(77)90390-6. [DOI] [PubMed] [Google Scholar]
- 31.George GN, George SJ, Pickering IK. EXAFSPAK. Stanford Synchrotron Radiation Laboratory; 1998. [Google Scholar]
- 32.Rehr JJ, Albers RC. Theoretical approaches to x-ray absorption fine structure. Reviews of Modern Physics. 2000;72:621–654. [Google Scholar]
- 33.Albrecht AG, Peuckert F, Landmann H, Miethke M, Seubert A, Marahiel MA. Mechanistic characterization of sulfur transfer from cysteine desulfurase SufS to the iron-sulfur scaffold SufU in Bacillus subtilis. FEBS Lett. 2011;585:465–470. doi: 10.1016/j.febslet.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 35.Collet JF, D'Souza JC, Jakob U, Bardwell JC. Thioredoxin 2, an oxidative stress-induced protein, contains a high affinity zinc binding site. J Biol Chem. 2003;278:45325–45332. doi: 10.1074/jbc.M307818200. [DOI] [PubMed] [Google Scholar]
- 36.Jakob U, Eser M, Bardwell JC. Redox switch of hsp33 has a novel zinc-binding motif. J Biol Chem. 2000;275:38302–38310. doi: 10.1074/jbc.M005957200. [DOI] [PubMed] [Google Scholar]
- 37.Wu SP, Mansy SS, Cowan JA. Iron-sulfur cluster biosynthesis. Molecular chaperone DnaK promotes IscU-bound [2Fe-2S] cluster stability and inhibits cluster transfer activity. Biochemistry. 2005;44:4284–4293. doi: 10.1021/bi0483007. [DOI] [PubMed] [Google Scholar]
- 38.Mansy SS, Wu G, Surerus KK, Cowan JA. Iron-sulfur cluster biosynthesis. Thermatoga maritima IscU is a structured iron-sulfur cluster assembly protein. J Biol Chem. 2002;277:21397–21404. doi: 10.1074/jbc.M201439200. [DOI] [PubMed] [Google Scholar]
- 39.Luttringer F, Mulliez E, Dublet B, Lemaire D, Fontecave M. The Zn center of the anaerobic ribonucleotide reductase from E. coli. J Biol Inorg Chem. 2009;14:923–933. doi: 10.1007/s00775-009-0505-9. [DOI] [PubMed] [Google Scholar]
- 40.Shimomura Y, Kamikubo H, Nishi Y, Masako T, Kataoka M, Kobayashi Y, Fukuyama K, Takahashi Y. Characterization and crystallization of an IscU-type scaffold protein with bound [2Fe-2S] cluster from the hyperthermophile, Aquifex aeolicus. Journal of biochemistry. 2007;142:577–586. doi: 10.1093/jb/mvm163. [DOI] [PubMed] [Google Scholar]
- 41.Johnson DC, Unciuleac MC, Dean DR. Controlled expression and functional analysis of iron-sulfur cluster biosynthetic components within Azotobacter vinelandii. J Bacteriol. 2006;188:7551–7561. doi: 10.1128/JB.00596-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldsmith-Fischman S, Kuzin A, Edstrom WC, Benach J, Shastry R, Xiao R, Acton TB, Honig B, Montelione GT, Hunt JF. The SufE sulfur-acceptor protein contains a conserved core structure that mediates interdomain interactions in a variety of redox protein complexes. J Mol Biol. 2004;344:549–565. doi: 10.1016/j.jmb.2004.08.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.