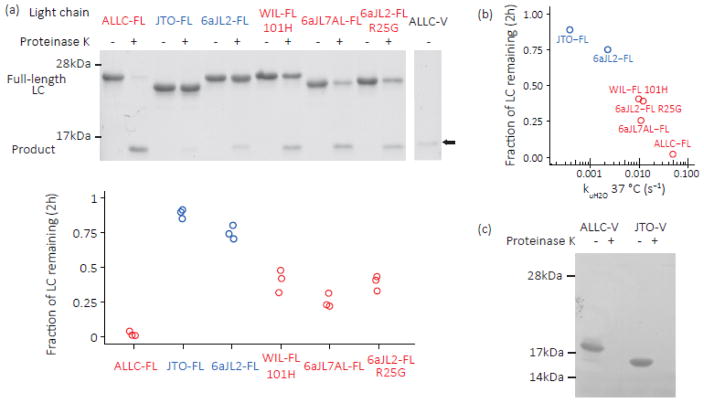
Fig. 8. Kinetically unstable amyloidogenic LC dimers are susceptible to endoproteolysis.
LCs were incubated with proteinase K at 37 °C, pH 7.4, then quenched with phenylmethyl sulfonyl fluoride (PMSF, 5 mM). (a) SDS-PAGE gels of purified, recombinant full-length LCs (10 μM monomer equivalent) incubated with proteinase K (100 nM) for 2 h at 37 °C in PBS buffer. Quantitation is shown below (n=3). Patient-derived amyloidogenic LC sequences (ALLC-FL, WIL-FL and 6aJL7AL-FL; red) are cleaved more readily than the non-amyloidogenic JTO-FL and 6aJL2-FL (blue), while the R25G mutation in 6aJL2-FL (red) increases its proteolytic susceptibility. LCs are initially cleaved into fragments the size of an isolated Ig domain; ALLC-V is shown in the right-most lane for reference (arrow). The product was identified as the C-domain by mass spectrometry (Fig. S5). (b) Correlation between unfolding rate and extent of proteolysis at 37 °C. The mean of the measurements in (a) is shown. (c) ALLC-V and JTO-V are completely proteolyzed by 30 min incubation with proteinase K (n=3).