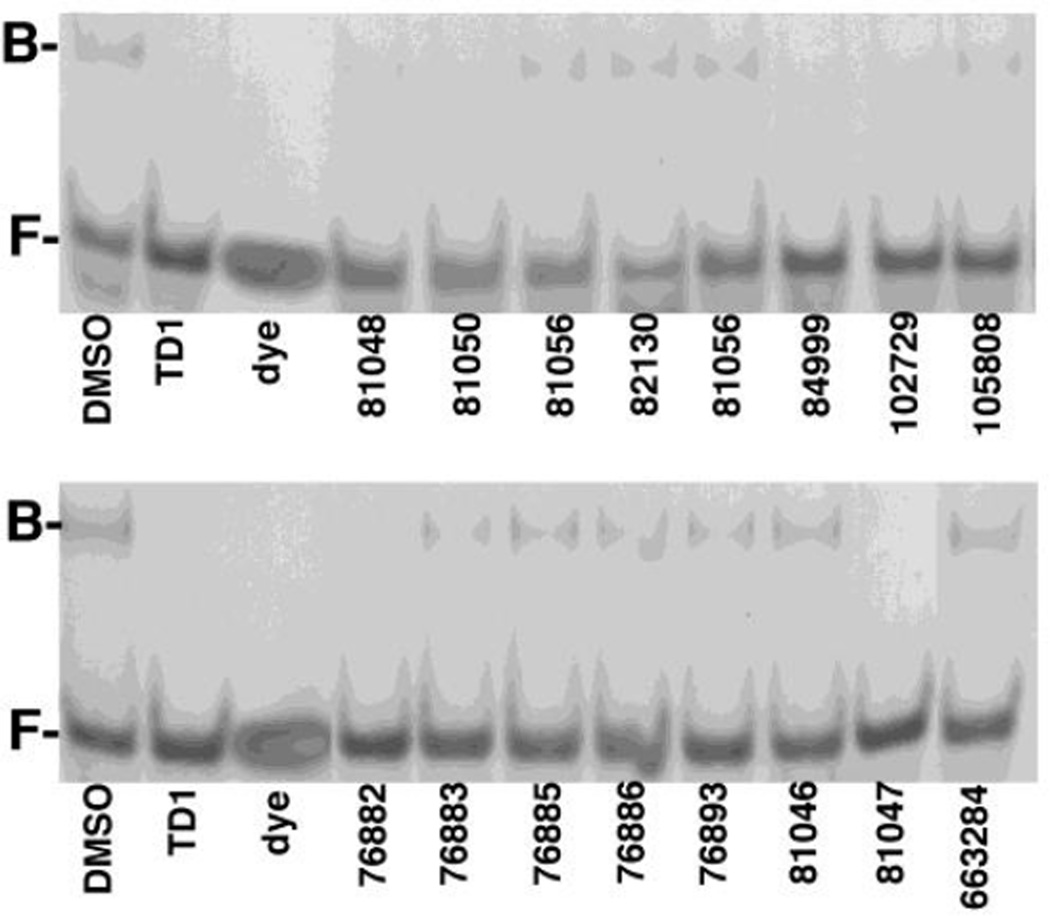
Figure 2. Inhibition of HIV-1 MA binding to RNA.
Electrophoretic mobility shift assays were performed to assess compound effects on HIV-1 MA binding to the Sel15 RNA oligonucleotide. MA (15 µM) samples were preincubated with 100 µM of the indicated compounds, then incubated with 15 µM RNA, after which free (F) and bound (B) RNAs were separated by electrophoresis and visualized by staining with Stains-All. Note that TD1 2-(2-chloro-6-methylphenyl)-4-(cyclopropylmethyl)-1,2,4-thiadiazolane-3,5-dione) was used as a postitive control for inhibition of MA-RNA binding, and that "dye" indicates a dyeonly lane, used to monitor electrophoresis.