Table 2. Structure–Activity Relationships for 3- and 4-Position Phenyl Modifications on Trisubstituted Hydroxylactamsa.
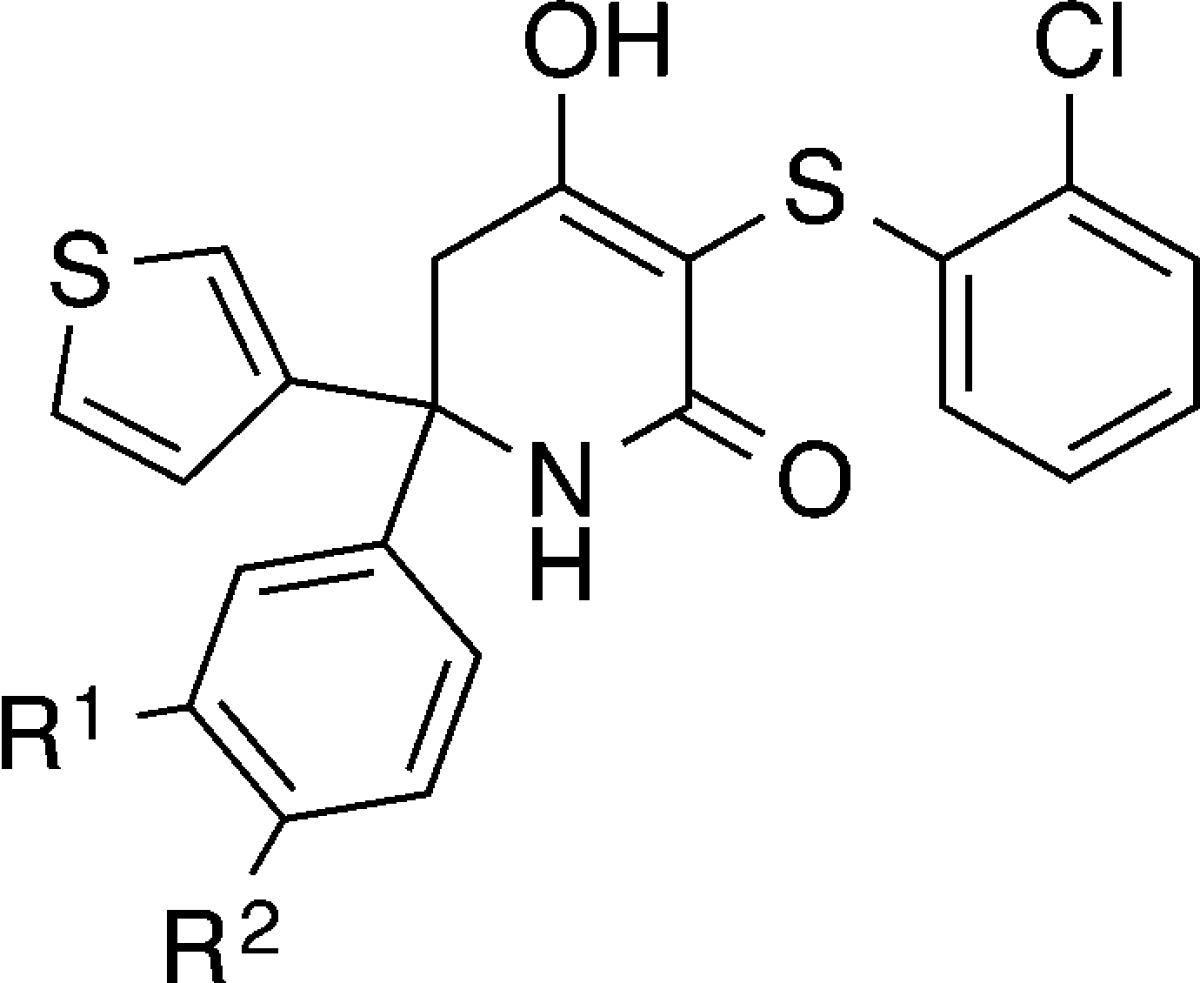
| compd | R1 | R2 | LDHA IC50 (μM) | LDHB IC50 (μM) |
|---|---|---|---|---|
| 15 | 1-morpholino | H | 0.080 | 0.25 |
| 16 | NH-THP | H | 0.009 | 0.16 |
| 17 | NH-cyclohexyl | H | 0.004 | 0.077 |
| 18 | NH-(4-F)Ph | H | 0.003 | 0.035 |
| 19 | O-THP | H | 0.069 | 0.66 |
| 20 | O-(4-F)Ph | H | 0.037 | 0.19 |
| 21 | H | cyclohexyl | 0.025 | 0.18 |
| 22 | H | 1-piperazinyl | 0.052 | 0.78 |
| 23 | H | 1-(4-oxetane)piperazinyl | 0.047 | 0.39 |
| 24 | H | 1-(4-OMe)piperidinyl | 0.016 | 0.075 |
| 25 | H | 1-piperidinyl | 0.009 | 0.049 |
| 26 | H | 1-pyrrolidinyl | 0.007 | 0.050 |
| 27 | H | 1-(4-acetyl)piperazinyl | 0.005 | 0.035 |
| 28 | H | 1-morpholino | 0.009 | 0.030 |
| 29-R | H | 1-morpholino | 0.003 | 0.005 |
| 30-S | H | 1-morpholino | 0.055 | 0.14 |
Compounds are racemic mixtures, except where indicated.
