Table 4. sPLA2 Potency and Optimization Parameters for Compounds 4–9.
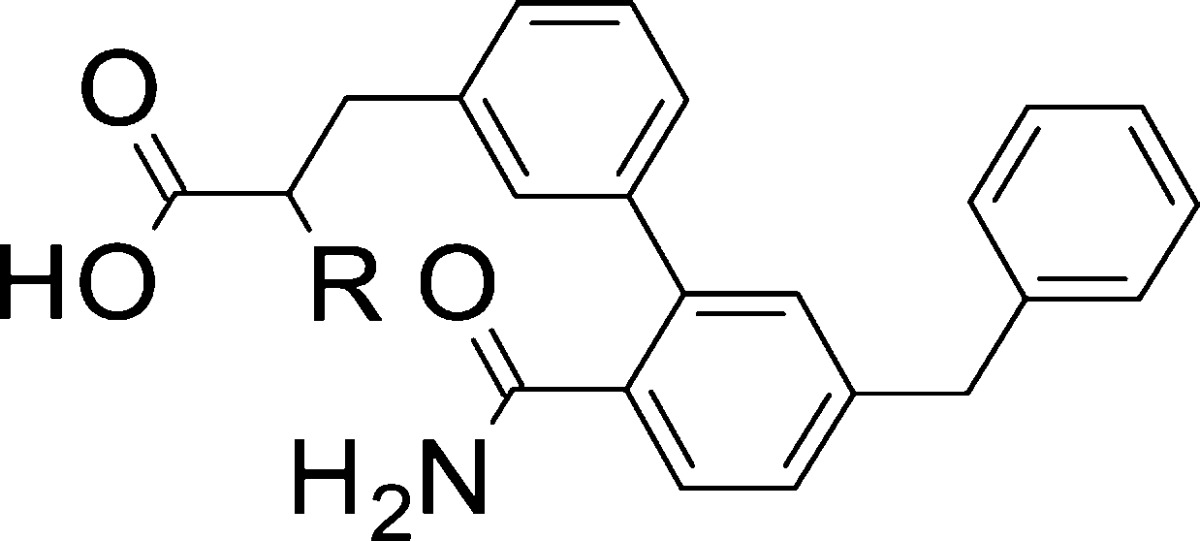
| entry | R | sPLA2-IIa IC50 (μM)a | sPLA2-V IC50 (μM)a | sPLA2-X IC50 (μM)a | plasma ICu,50 (nM)b | HEP Clint (μL/min/10–6 cells)c | OATP1B1 IC50 (μM)d |
|---|---|---|---|---|---|---|---|
| 4 | H | 0.012 | 0.36 | 0.28 | 7 | 5.2 | 2 |
| 7 | Me | 0.011 | 0.07 | 0.75 | 1 | 9.6 | NAe |
| 8 | Et | 0.021 | 0.07 | 0.43 | 0.8 | 25 | NDf |
| 9 | CyPr | 0.018 | 0.25 | 0.58 | 0.9 | 21 | NDf |
| (S)-7 | (S)-Me | 0.038 | 1.2 | 3.8 | ND | 9.3 | NDf |
| (R)-7 | (R)-Me | 0.010 | 0.04 | 0.4 | 0.1 | 12 | NAe |
Mean of at least two experiments. Experimental errors within 20% of value.
Calculated as Plasma sPLA2 IC50 (μM) × unbound fraction in human plasma (Fu)/100.
Intrinsic clearance of test compounds after incubation with human hepatocytes.
Inhibition of pivastatin uptake to HEK293 cells transfected with human OATP1B1.
Not active at maximum tested concentration (25 μM).
Not determined.
