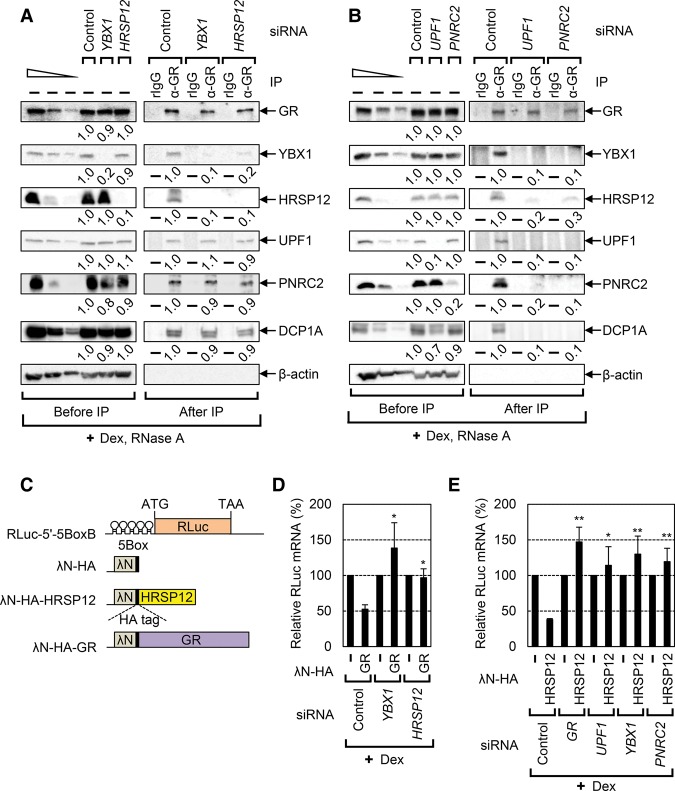
Figure 5.
Sequential recruitment of GMD factors is involved in the formation of the active GMD complex. (A) Immunoprecipitation of endogenous GR using extracts of HEK293T cells depleted of either YBX1 or HRSP12. The cells were treated with Dex for 1 h before cell harvest. Total cell extracts were treated with RNase A to rule out RNA-mediated indirect interactions. Immunoprecipitations were performed using either α-GR antibody or, as a control, rabbit IgG (rIgG). The results are representative of two independently performed experiments. The levels of coimmunoprecipitated proteins were normalized to the level of immunoprecipitated GR. The normalized levels obtained from GR immunoprecipitation using undepleted cells were arbitrarily set to 1.0 (see also Supplemental Table S3 for details of quantitation). (B) Immunoprecipitation of endogenous GR using the extracts of the cells depleted of either UPF1 or PNRC2. This was performed as in A except that endogenous UPF1 or PNRC2 was down-regulated by siRNAs. n = 2. (C) A schematic diagram of the λN/5Box system. (D,E) GMD of the reporter mRNAs elicited by tethered GR (D) or HRSP12 (E). Cells were depleted of the indicated proteins using specific siRNAs, and then the tethering reporter and effector were transiently expressed. The cells were treated with Dex for 30 min before cell harvest. n = 3. (**) P < 0.01; (*) P < 0.05.