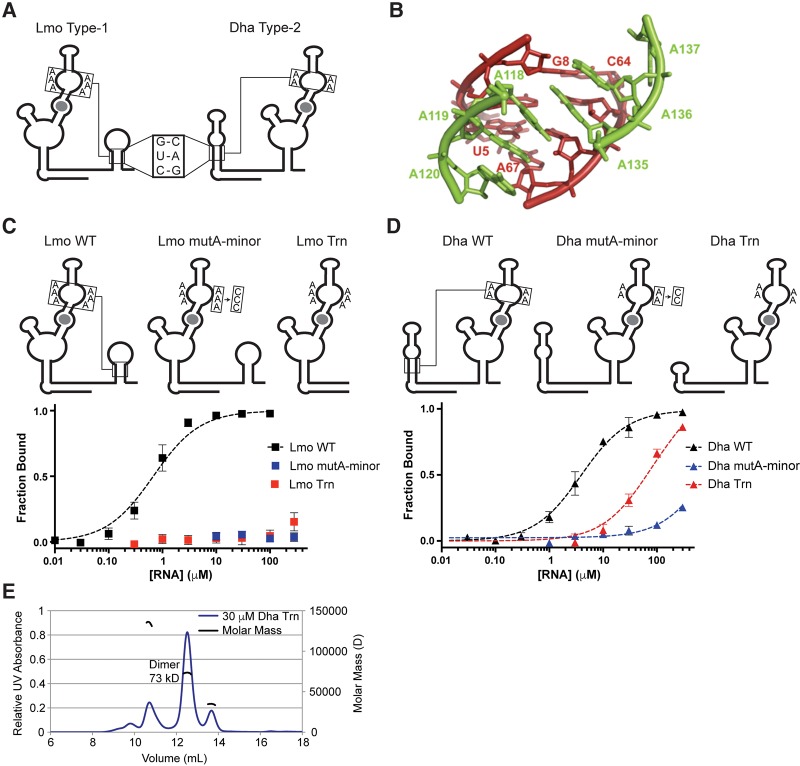
FIGURE 4.
Singlet riboswitches require conserved A-minor tertiary interactions with an adjacent “ghost-aptamer” domain. (A) Diagrams highlighting the conserved helical regions and A-rich bulges in the Lmo and Dha riboswitches. (B) Structural model of the conserved A-minor interaction from the F. nucleatum tandem riboswitch (Butler et al. 2011). (C,D) Diagrams and glycine-binding curves for singlet mutants with disrupted A-minor interactions. (E) MALS analysis of Dha Trn refolded in the presence of saturating glycine, showing that the major species is an alternative dimer conformation.