Abstract
Aim
To compare efficacy and safety of new insulin glargine 300 U/ml (Gla‐300) with that of insulin glargine 100 U/ml (Gla‐100) in Japanese adults with type 1 diabetes.
Methods
The EDITION JP 1 study (NCT01689129) was a 6‐month, multicentre, open‐label, phase III study. Participants (n = 243) were randomized to Gla‐300 or Gla‐100 while continuing mealtime insulin. Basal insulin was titrated with the aim of achieving a fasting self‐monitored plasma glucose target of 4.4–7.2 mmol/l. The primary endpoint was change in glycated haemoglobin (HbA1c) over 6 months. Safety measures included hypoglycaemia and change in body weight.
Results
Gla‐300 was non‐inferior to Gla‐100 for the primary endpoint of HbA1c change over the 6‐month period {least squares [LS] mean difference 0.13 % [95 % confidence interval (CI) −0.03 to 0.29]}. The annualized rate of confirmed (≤3.9 mmol/l) or severe hypoglycaemic events was 34 % lower with Gla‐300 than with Gla‐100 at night [rate ratio 0.66 (95 % CI 0.48–0.92)] and 20 % lower at any time of day [24 h; rate ratio 0.80 (95 % CI 0.65–0.98)]; this difference was most pronounced during the first 8 weeks of treatment. Severe hypoglycaemia was infrequent. The basal insulin dose increased in both groups (month 6 dose: Gla‐300 0.35 U/kg/day, Gla‐100 0.29 U/kg/day). A between‐treatment difference in body weight change over 6 months favouring Gla‐300 was observed [LS mean difference −0.6 kg (95 % CI −1.1 to −0.0); p = 0.035]. Adverse event rates were comparable between the groups.
Conclusions
In Japanese adults with type 1 diabetes using basal plus mealtime insulin, less hypoglycaemia was observed with Gla‐300 than with Gla‐100, particularly during the night, while glycaemic control did not differ.
Keywords: basal insulin, glycaemic control, insulin analogues, phase III study, randomised trial, type 1 diabetes
Introduction
Insulin‐based therapy remains the mainstay of treatment for type 1 diabetes worldwide, with most people receiving a combination of basal plus mealtime insulin or continuous subcutaneous insulin infusion 1, 2, 3. The once‐daily, long‐acting basal insulin analogue insulin glargine 100 U/ml [Gla‐100 (Lantus®; Sanofi, Paris, France)] was licensed in Japan in 2003 4 and has been shown to achieve glycaemic control similar to or greater than that of human neutral protamine Hagedorn (NPH) insulin 5, 6, accompanied by numerically fewer mild‐to‐moderate hypoglycaemic events 5 and less severe hypoglycaemia 6. Nevertheless, despite the available treatment options, >45 % of people with diabetes worldwide do not achieve their glycated haemoglobin (HbA1c) targets 7, 8, 9. There is considerable interest in further improving type 1 diabetes treatment with a basal insulin that maintains glycaemic control while minimizing the risk of hypoglycaemia and allowing flexibility of injection times 2, 10, 11. Pharmacokinetic (PK) and pharmacodynamic (PD) studies with new insulin glargine 300 U/ml (Gla‐300) indicate that it has the potential to address these clinical needs 12, 13, 14. In a single‐dose study in Japanese people with type 1 diabetes, Gla‐300 showed more stable and prolonged PK and PD profiles compared with Gla‐100, with tight blood glucose control maintained for up to 36 h 14. This was also observed in a European population 14 and is consistent with the more stable and prolonged PK and PD profiles observed at steady state with Gla‐300 in a European population 13.
The phase IIIa EDITION programme has shown that Gla‐300 is as effective as Gla‐100 in achieving glucose control in multinational populations with type 1 or 2 diabetes 15, 16, 17, 18, 19 without raising any safety concerns. In Western populations with type 2 diabetes, it was shown that glycaemic control was accompanied by a reduction in hypoglycaemia risk with Gla‐300 versus Gla‐100 15, 17, 19. In a multinational type 1 diabetes population (EDITION 4), comparable glycaemic control was observed, but with no difference in annualized rates of hypoglycaemia, except during the first 8 weeks of treatment, when the rate of nocturnal confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia was lower with Gla‐300 than with Gla‐100 16. In addition, a study in people with type 1 diabetes that used continuous glucose monitoring showed more stable 24‐h glucose levels and lower glucose variability, with lower annualized rates of nocturnal confirmed [<3.0 mmol/l (<54 mg/dl)] or severe hypoglycaemia with Gla‐300 than with Gla‐100 20. In both of these studies in type 1 diabetes, the same efficacy and safety was observed with Gla‐300, regardless of injection time (morning versus evening) 16, 20. Gla‐300 was licensed in the USA, Europe and Japan in 2015 (as Toujeo® in the USA and Europe and as Lantus® XR in Japan). In the present study, we investigated the efficacy and safety of Gla‐300 versus Gla‐100 in Japanese adults with type 1 diabetes already using basal and mealtime insulin.
Materials and Methods
Research Design
The EDITION JP1 study (NCT01689129) was a multicentre, randomized, open‐label, two‐arm, parallel‐group, phase III study in Japanese participants with type 1 diabetes. The study comprised a 2‐week screening phase and a 6‐month, main treatment period, followed by a preplanned 6‐month extension period. The results from the main 6‐month treatment period are reported in the present paper. The protocol was amended after study commencement to include an independent review of all hypoglycaemic events reported as severe and/or as serious adverse events (SAEs) by a Severe Hypoglycaemia Review Board to ensure consistency across the EDITION studies. The appropriate ethics committees approved the study, which was conducted in accordance with Good Clinical Practice 21 and the Declaration of Helsinki 22. Written informed consent was obtained from all participants.
Participants
Participants were recruited as outpatients at 22 centres in Japan. Adults ≥18 years with type 1 diabetes receiving basal and mealtime insulin for ≥1 year with HbA1c ≥7.0 and ≤10.0 % (≥53 and ≤86 mmol/mol) at screening were included. Key exclusion criteria were: unstable insulin dose (±20 % total basal insulin dose) in the previous 30 days; use of premixed insulin, human regular insulin as mealtime insulin and/or any antihyperglycaemic drugs other than basal insulin and mealtime rapid‐acting insulin analogues within 3 months; use of an insulin pump within 6 months; any contraindication for use of insulin glargine as defined by the product labelling in Japan; severe hypoglycaemia resulting in coma/seizures or hospitalization for diabetic ketoacidosis within 6 months.
Randomization and Masking
Participants were randomized (1 : 1) to Gla‐300 or Gla‐100, stratified by HbA1c at screening visit [<8.0 or ≥8.0 % (<64 or ≥64 mmol/mol)]. Owing to differences between insulin injection devices and injection volumes, the study was open‐label; however, efficacy variables were assessed based on anonymized samples at the central laboratory.
Interventions
Participants received once‐daily subcutaneous injections of Gla‐300 [using a modified TactiPen® injector (Haselmeier GmbH, Zürich, Switzerland)] or Gla‐100 [using a SoloSTAR® injector (Sanofi)], at the same time each evening (between pre‐dinner and bedtime). The initial daily dose of Gla‐300 or Gla‐100 was equal to the total daily basal insulin dose on the day preceding the baseline visit for those previously receiving Gla‐100 (once or twice daily), NPH insulin or insulin detemir once daily, or 20 % less for those previously receiving NPH insulin or insulin detemir more than once daily. Gla‐300 or Gla‐100 was titrated to a fasting (preprandial) self‐monitored plasma glucose (SMPG) target of 4.4–7.2 mmol/l (80–130 mg/dl). Basal insulin dose titration was performed once weekly, and no more than every 3–4 days when more frequent adjustments were required. Visits (either by phone or on‐site) were scheduled once weekly up to week 12, then on weeks 17, 22 and 26. Additional visits could be scheduled to discuss dose adjustments if required. At the discretion of the investigator, participants could be allowed to adjust their basal insulin dose without prior consultation with site personnel. The basal insulin dose was increased if the median of the fasting (pre‐breakfast) SMPG over the last 3 days was >7.2 mmol/l (>130 mg/dl) with no evidence of relevant hypoglycaemia. Gla‐300 and Gla‐100 were increased by >10 and 10 % of the daily dose, respectively, in ranges of 1.5–4.5 and 1.0–4.0 U/day. If fasting (preprandial) SMPG was <4.4 mmol/l (<80 mg/dl) or if relevant hypoglycaemia occurred, the dose was reduced by 1.5 or 1.0 U/day for Gla‐300 and Gla‐100, respectively, at the investigator's discretion. Upward titration was stopped for 1 week and the dose decreased at the investigator's discretion if fasting (preprandial) SMPG was <3.3 mmol/l (<60 mg/dl) or an episode of severe hypoglycaemia (requiring assistance) was reported without an adequate explanation for the event (such as missing a meal or heavy exercise). Participants continued mealtime insulin during the study, administered according to approved labelling in Japan and titrated to achieve glycaemic control after basal insulin doses had been optimized; mealtime dose could be reduced while basal insulin doses were increased to avoid daytime hypoglycaemia.
Outcomes
The primary endpoint was change in HbA1c from baseline to month 6. Secondary efficacy endpoints included change in laboratory‐measured fasting plasma glucose (FPG), pre‐injection SMPG [measured directly before the time of basal insulin administration; this could coincide with the time of an eight‐point SMPG measurement (i.e. bedtime), in which case one value was to be recorded for both measures], mean overall eight‐point SMPG profiles (03:00 h, before breakfast, 2 h after breakfast, before lunch, 2 h after lunch, before dinner, 2 h after dinner, bedtime), change in daily basal insulin dose and daily mealtime insulin dose from baseline to month 6. The percentages of participants reaching HbA1c <7.0 % (<53 mmol/mol) with and without experiencing hypoglycaemic events were also examined. Exploratory efficacy endpoints included the percentage of participants with laboratory‐measured FPG <5.6 mmol/l (<100 mg/dl) at month 6. All hypoglycaemic events were recorded based on American Diabetes Association definitions 23 according to the time of day that they occurred [any time of day (24 h) or nocturnal (00:00–05:59 h)] and by study period (full 6‐month study period, baseline to week 8 and week 9 to month 6). The categories included: documented symptomatic hypoglycaemia [symptomatic events with SMPG ≤3.9 mmol/l (≤70 mg/dl)]; asymptomatic hypoglycaemia [events without symptoms confirmed by SMPG ≤3.9 mmol/l (≤70 mg/dl)]; severe hypoglycaemia (events requiring assistance from another person); confirmed or severe hypoglycaemia (all documented symptomatic, asymptomatic and severe events); probable symptomatic hypoglycaemia (symptoms of hypoglycaemia without SMPG determination). The occurrence of hypoglycaemic events at a lower <3.0 mmol/l (<54 mg/dl) threshold was also analysed. Other clinical and safety measurements included body weight, injection site reactions, hypersensitivity reactions and other AEs.
Data Analysis and Statistics
A sample size of 240 participants (N = 120 for both groups) was estimated to give 90 % power for the upper confidence limit of the mean difference in change in HbA1c between treatment groups not to exceed 0.4 % (4.4 mmol/mol), assuming the standard deviation (s.d.) was 0.9 %, for a true difference of 0.0 %, and that all participants were evaluable. Non‐inferiority of Gla‐300 versus Gla‐100 was shown if the upper limit of the two‐sided confidence interval (CI) for the difference in mean change in HbA1c versus baseline was lower than the predefined non‐inferiority margin of 0.4 % (4.4 mmol/mol).
Efficacy endpoints were analysed using the modified intention‐to‐treat population (all randomized participants who received ≥1 dose of study treatment, and had both a baseline assessment and ≥1 post‐baseline assessment for any efficacy variable). Safety endpoints were analysed descriptively using the safety population (all randomized participants exposed to ≥1 dose of the study treatment). Hypoglycaemia was analysed as the percentage of participants experiencing ≥1 event and the number of events per participant‐year. Between‐treatment differences in body weight and annualized rates of hypoglycaemia were analysed post hoc. Further details on statistical analyses are provided in Appendix S1.
Results
Participants
Eligible participants were randomized (N = 122, Gla‐300; N = 121, Gla‐100) between October 2012 and October 2013 (Figure 1). All randomized participants received study treatment. Baseline characteristics were not different between the two groups (Table 1). The discontinuation rate was 4.1 % for the Gla‐300 group and 3.3 % for the Gla‐100 group.
Figure 1.
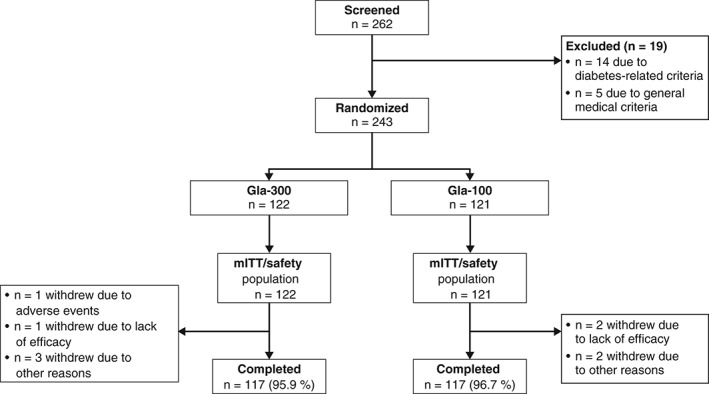
Flow of participants through the main 6‐month period of the EDITION JP 1 study [modified intention‐to‐treat (mITT) and safety populations]. Gla‐100, insulin glargine 100 U/ml; Gla‐300, insulin glargine 300 U/ml.
Table 1.
Baseline characteristics of the randomized population.
| Gla‐300 | Gla‐100 | All | |
|---|---|---|---|
| Characteristic | N = 122 | N = 121 | N = 243 |
| Age, mean (s.d.) years | 44.1 (13.9) | 46.3 (15.3) | 45.2 (14.6) |
| Male gender, n (%) | 56 (45.9) | 56 (46.3) | 112 (46.1) |
| Duration of type 1 diabetes, mean (s.d.) years | 12.2 (8.6) | 13.9 (9.0) | 13.0 (8.8) |
| Body weight, mean (s.d.) kg | 63.9 (11.6) | 61.0 (11.8) | 62.5 (11.7) |
| BMI, mean (s.d.) kg/m2 | 23.8 (3.9) | 23.2 (3.3) | 23.5 (3.6) |
| HbA1c | |||
| mean (s.d.) mmol/mol | 64.6 (7.0) | 64.7 (8.1) | 64.7 (7.5) |
| mean (s.d.) % | 8.06 (0.64) | 8.07 (0.74) | 8.07 (0.69) |
| Previous basal insulin type, n (%) | |||
| Insulin glargine | 110 (90.2) | 109 (90.1) | 219 (90.1) |
| NPH insulin | 0 | 1 (0.8) | 1 (0.4) |
| Insulin detemir | 12 (9.8) | 11 (9.1) | 23 (9.5) |
| Previous basal insulin daily injection number, n (%) | |||
| Once daily | 84 (68.9) | 82 (67.8) | 166 (68.3) |
| Twice daily | 38 (31.1) | 39 (32.2) | 77 (31.7) |
| Previous daily basal insulin dose, mean (s.d.) | |||
| U/kg/day | 0.28 (0.12)* | 0.30 (0.13)† | 0.29 (0.13) |
| U/day | 18.2 (9.2)* | 18.5 (9.7)† | 18.3 (9.4) |
| Previous daily mealtime insulin dose, mean (s.d.) | |||
| U/kg/day | 0.45 (0.18) | 0.45 (0.16) | 0.45 (0.17) |
| U/day | 29.1 (14.1) | 27.2 (11.7) | 28.2 (13.0) |
| Previous daily total insulin dose, mean (s.d.) | |||
| U/kg/day | 0.73 (0.26)* | 0.74 (0.23)† | 0.74 (0.24) |
| U/day | 47.1 (21.2)* | 45.6 (17.8)† | 46.4 (19.6)‡ |
BMI, body mass index; Gla‐100, insulin glargine 100 U/ml; Gla‐300, insulin glargine 300 U/ml; HbA1c, glycated haemoglobin; NPH, neutral protamine Hagedorn; s.d., standard deviation.
N = 116.
N = 119.
N = 235.
Glycaemic Control
At month 6, the mean HbA1c level had decreased by 0.30 % (3.3 mmol/mol) with Gla‐300 versus 0.43 % (4.7 mmol/mol) with Gla‐100 (Figure 2A and Table S1). The least squares (LS) mean difference was 0.13 % [95 % confidence interval (CI) −0.03 to 0.29], demonstrating non‐inferiority of Gla‐300 versus Gla‐100. No between‐treatment differences were observed in change from baseline to month 6 in laboratory‐measured FPG (Table S1 and Figure 2B). The percentages of people receiving Gla‐300 and Gla‐100 who achieved HbA1c <7.0 % (<53 mmol/mol) were 15.6 % (19/122) and 20.0 % (24/120), respectively (Table S1).
Figure 2.
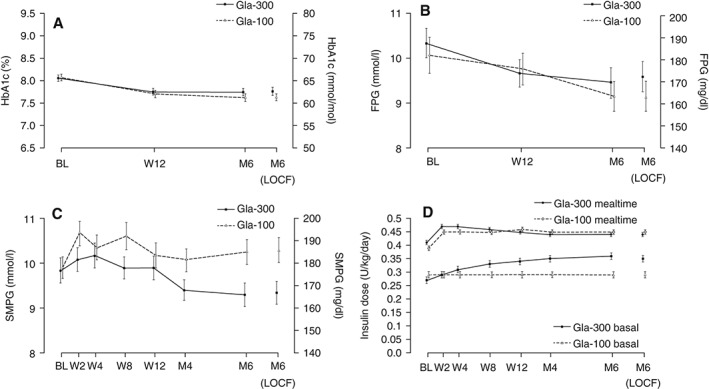
Clinical measures across the 6‐month study period in the modified intention‐to‐treat (mITT) population: (A) glycated haemoglobin (HbA1c), (B) laboratory‐measured fasting plasma glucose (FPG), (C) average pre‐injection self‐monitored plasma glucose (SMPG) profile and (D) daily basal and mealtime insulin dose. Data are shown as mean ± standard error. Gla‐100, insulin glargine 100 U/ml; Gla‐300, insulin glargine 300 U/ml; BL, baseline; W, week; M, month; LOCF, last observation carried forward.
At month 6, SMPG was consistently lower at all time points (demonstrated by average eight‐point SMPG profiles) in the Gla‐300 group compared with baseline, whereas there was no consistent trend in the Gla‐100 group (Figure 3). At month 6, mean pre‐dinner SMPG was significantly lower with Gla‐300 [8.4 mmol/l (151.7 mg/dl)] versus Gla‐100 [10.0 mmol/l (180.3 mg/dl); LS mean difference −1.6 (95 % CI −2.8 to −0.3) mmol/l or −28.1 mg/dl (95 % CI −50.1 to −6.1)]. Glycaemic control was notably better with Gla‐300 than Gla‐100 from pre‐dinner to bedtime at month 6 (Figure 3 and Table S1). At month 6, average pre‐injection SMPG was also significantly lower with Gla‐300 [9.3 mmol/l (166.8 mg/dl)] versus Gla‐100 [10.3 mmol/l (185.8 mg/dl); LS mean difference −1.0 mmol/l (95 % CI −1.8 to −0.3) or −18.5 mg/dl (95 % CI −32.0 to −5.0); Figure 2C and Table S1].
Figure 3.
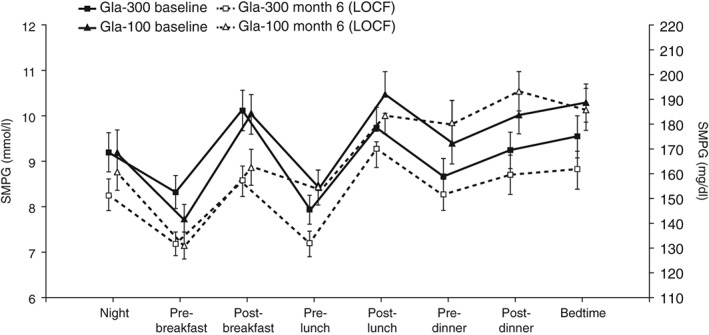
Mean eight‐point self‐monitored plasma glucose (SMPG) profiles at baseline and month 6 (LOCF; modified intention‐to‐treat population). Data are shown as mean ± standard error. Gla‐100, insulin glargine 100 U/ml; Gla‐300, insulin glargine 300 U/ml; LOCF, last observation carried forward.
Insulin Dose
The mean (s.d.) total insulin dose at month 6 was 0.79 (0.25) U/kg/day (basal 0.35 U/kg/day; mealtime 0.44 U/kg/day) for the Gla‐300 group and 0.74 (0.22) U/kg/day (basal 0.29 U/kg/day; mealtime 0.45 U/kg/day) for the Gla‐100 group (Figure 2D). This corresponds to a mean (s.d.) total insulin dose of 50.7 (20.4) U/day (basal 23.0 U/day; mealtime 28.0 U/day) for the Gla‐300 group and 46.0 (17.6) U/day (basal 18.2 U/day; mealtime 27.8 U/day) for the Gla‐100 group.
Hypoglycaemia
Hypoglycaemia was reported in 119 participants (4622 events) in the Gla‐300 group and in 118 participants (5696 events) in the Gla‐100 group. Overall hypoglycaemia rates were lower with Gla‐300 than with Gla‐100 both at any time (24 h) and during the night (00:00–05:59 h; Tables S2 and S3).
Confirmed or Severe Hypoglycaemia
Hypoglycaemia at any time of day
The cumulative mean number of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic events per participant at any time (24 h) was lower with Gla‐300 than with Gla‐100 throughout the study (Figure 4A). Annualized rates of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia were lower with Gla‐300 versus Gla‐100 over 6 months (Figure 4B and Table S2). In addition, numerically fewer confirmed [<3.0 mmol/l (<54 mg/dl)] or severe hypoglycaemic events were reported between baseline and month 6 by Gla‐300‐treated participants (Figure 4E and Table S2).
Figure 4.
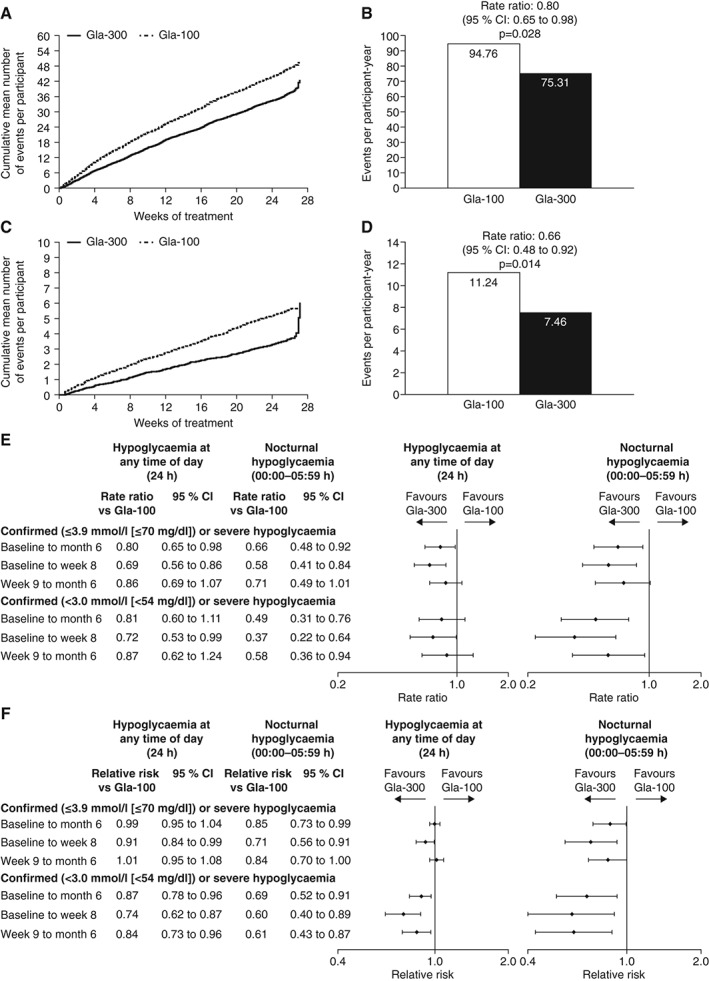
Occurrence of confirmed or severe hypoglycaemic events during the 6‐month study period (safety population): (A) cumulative mean number of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe events per participant at any time (24 h) and (B) events per participant‐year; (C) cumulative mean number of nocturnal (00:00–05:59 h) confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe events per participant and (D) events per participant‐year; (E) ratio of event rates and (F) relative risk, during the night (00:00–05:59 h) and at any time (24 h). CI, confidence interval.
Almost all participants experienced confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia during the study, with no difference between treatment groups (Figure 4F and Table S3). Fewer participants in the Gla‐300 group than the Gla‐100 group experienced confirmed or severe hypoglycaemia at the stricter <3.0 mmol/l (<54 mg/dl) threshold (Figure 4F and Table S3).
Nocturnal hypoglycaemia
The cumulative mean number of nocturnal (00:00–05:59 h) confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic events per participant was lower with Gla‐300 than with Gla‐100 throughout the study period (Figure 4C). The rapid increase during the last 8 days of the main 6‐month treatment period in the Gla‐300 group is an artefact caused by the very low number of participants exposed to treatment during this time who experienced only one event on each of days 187, 189 and 190. Annualized rates were lower with Gla‐300 than with Gla‐100 at both thresholds (Figure 4D and E and Table S2). Similarly, the percentage of participants experiencing ≥1 nocturnal confirmed or severe hypoglycaemic event over 6 months was lower with Gla‐300 than with Gla‐100 at both thresholds (Figure 4F and Table S3).
Distribution of Hypoglycaemia Over 24 h
The majority of hypoglycaemic events, regardless of category, occurred between 06:00 and 23:59 h (4155 and 5011 events with Gla‐300 and Gla‐100, respectively). The annualized rate of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe events over 6 months was not only similar or lower with Gla‐300 versus Gla‐100 during the night, but also up to 14:59 h (Figure S1).
Other Hypoglycaemia Definitions
Similar results to those described for confirmed or severe hypoglycaemia at both thresholds were seen across other definitions of hypoglycaemia (Tables S2 and S3). For both rates and risk of hypoglycaemia, the reduction in favour of Gla‐300 was more pronounced in the first 8 weeks for all analysed definitions (Tables S2 and S3).
Severe Hypoglycaemia
The percentage of participants experiencing severe hypoglycaemia was 5.7 % (7/122) with Gla‐300 and 9.9 % (12/121) with Gla‐100; annualized rates were 0.32 versus 0.22 events per participant‐year (Tables S2 and S3).
Body Weight
Change in body weight over 6 months is shown in Figure S2. LS mean change (standard error) was −0.1 (0.2) kg with Gla‐300 and 0.4 (0.2) kg with Gla‐100 [LS mean difference −0.6 kg (95 % CI −1.1 to −0.0); p = 0.035].
Adverse Events
A total of 62 % (76/122) and 64 % (78/121) of participants in the Gla‐300 and Gla‐100 groups experienced treatment‐emergent AEs. The most commonly reported were infections and infestations, skin and connective tissue disorders, and gastrointestinal disorders (Table S4). Treatment‐emergent AEs considered related to treatment were reported by three and two participants in the Gla‐300 and Gla‐100 groups, respectively. Treatment‐emergent SAEs were reported by 2.5 % (3/122 with Gla‐300 and 3/121 with Gla‐100) of participants in each group. One participant receiving Gla‐300 permanently discontinued because of a spinal column stenosis, which was not considered to be related to study treatment. No injection site reactions were reported in either group. Hypersensitivity reactions were reported in 6.6 % (8/122) of participants in the Gla‐300 group and 11.6 % (14/121) of participants in the Gla‐100 group; none were considered to be serious or related to treatment or led to treatment discontinuation. No deaths occurred in either treatment group.
Discussion
In this first study of the EDITION programme investigating outcomes with Gla‐300 in a Japanese population with type 1 diabetes treated with basal and mealtime insulin, Gla‐300 was non‐inferior to Gla‐100 for the primary efficacy measure of change in HbA1c from baseline to month 6. Consistent with other EDITION studies (in type 1 and 2 diabetes in larger worldwide populations) 15, 16, 17, 19, no between‐treatment difference in HbA1c reduction was observed. This is similar to results observed in two studies comparing Gla‐100 and insulin degludec in multinational populations, which have also indicated no differences in HbA1c reduction between treatments 24, 25. Similar results between Gla‐300 and Gla‐100 were also found in other glycaemic response parameters, including laboratory‐measured FPG and the percentage of participants meeting HbA1c and FPG targets, as reported in other EDITION studies 15, 16, 17, 18, 19; however, between‐treatment differences were observed in average pre‐dinner, as well as in post‐dinner and bedtime SMPG. In addition, pre‐injection SMPG across this 6‐month study was lower with Gla‐300 than with Gla‐100. Because daily prandial insulin dose was identical between treatments, it is likely that the difference observed in pre‐dinner, post‐dinner and pre‐injection SMPG reflects more the differential effect of Gla‐300, with longer PK and PD profiles, versus Gla‐100 13, 14 than the prandial insulin given at lunch and dinner. This could be particularly relevant for the 30 % of participants who had switched from twice‐daily basal insulin.
The importance of balancing attainment of glycaemic control with risk of hypoglycaemia in diabetes management is reflected by guidelines from the Japanese Diabetes Society, which include an HbA1c target of <8.0 % for use in individual cases when treatment intensification is considered to be difficult because of the risk of hypoglycaemia 3; therefore, it is clinically relevant that the use of Gla‐300 in the present study was associated with a lower risk of hypoglycaemia at any time of day, and particularly during the night, compared with Gla‐100. The difference in frequency of events was not modest: the annualized rate of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia with Gla‐300 was 20 and 34 % lower than that for Gla‐100 for events occurring at any time (24 h) and at night (00:00–05:59 h), respectively. In addition, the difference in rates of hypoglycaemia favouring Gla‐300 was shown to extend beyond this predefined nocturnal period and into the daytime hours. The reduction in hypoglycaemia risk with Gla‐300 versus Gla‐100 may be attributable to the smoother, more even PK and PD profiles of Gla‐300 13, and the low within‐day variability in Gla‐300 exposure 12. It is notable that the difference between treatment groups in the occurrence of hypoglycaemia was seen most prominently between 06:00 and 08:59 h (Figure S1). This is potentially attributable to ascertainment bias, as participants may have been more likely to check their plasma glucose during this time than during the night.
The lower frequency of nocturnal hypoglycaemia in the Gla‐300 group was observed throughout the study period across the different definitions, but was particularly apparent during the first 8 weeks, when most of the increase in basal insulin dose occurred. This is consistent with results from a multinational population of people with type 1 diabetes in EDITION 4, in which a 31 % lower rate of nocturnal confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia was observed in the first 8 weeks with Gla‐300 versus Gla‐100 (although in EDITION 4 the between‐treatment difference was not maintained over the whole study period); however, the significantly lower rate of hypoglycaemia at any time of day (24 h) with Gla‐300 versus Gla‐100 in the present study is not reflected in EDITION 4, where no between‐treatment differences in rates at any time of day were observed 16. These contrasting results may be explained in part by differences in diet and lifestyle between the populations, which could alter the effect of exogenous insulin, and hence, risk of hypoglycaemia. In addition, half of the participants in EDITION 4 received their basal insulin dose in the morning and half in the evening, whereas all of the participants in the present study received their basal insulin dose during the evening. Significantly lower rates of nocturnal confirmed or severe hypoglycaemia were also observed with Gla‐300 than with Gla‐100 in the present study at the more stringent threshold [<3.0 mmol/l (<54 mg/dl)]. These are of particular interest in type 1 diabetes, in which the biochemical mechanisms that protect against hypoglycaemia are compromised 26. Similar results were observed in a continuous glucose monitoring study of people with type 1 diabetes, in which the annualized rate of similarly defined hypoglycaemia was lower with Gla‐300 than with Gla‐100 20.
As expected, given the wealth of clinical experience on the safety of Gla‐100 27, Gla‐300 was well tolerated with no reported injection site reactions or safety concerns. There was a small but statistically significant difference in body weight change between groups; however, the reason for this is not known. A slightly higher Gla‐300 dose was required, which was probably related to a lower 24‐h exposure versus Gla‐100 at equal doses, which may be attributable to increased residence time in, and slower absorption from, the smaller subcutaneous depot. Despite the slightly higher dose, fewer hypoglycaemic events and smaller weight changes were experienced by participants receiving Gla‐300 versus Gla‐100, showing that there were no clinical implications in terms of safety, consistent with other EDITION studies 15, 16, 17, 18, 19.
To date, the efficacy and safety of newer long‐acting insulin analogues in people with type 1 diabetes in Japan has only been reported for one other randomized controlled study, which evaluated insulin degludec versus insulin detemir administered once daily at bedtime in 66 adults with type 1 diabetes 28. The study showed that the use of insulin degludec was associated with similar glycaemic control and a lower risk of nocturnal hypoglycaemia versus insulin detemir; however, the differences in study design and methodology between these two studies, including the different control insulins, limit direct comparisons.
The present study extends previous observations regarding efficacy and safety of Gla‐300 in type 1 diabetes from multinational populations 16, 20 to Japanese adults. No difference in glycaemic control with Gla‐300 and Gla‐100 was confirmed, together with greater relative reductions in hypoglycaemic event rates with Gla‐300. The strengths of the present study include adequate statistical power to answer the questions posed, careful supervision of basal insulin titration and identification of hypoglycaemic events, and very high participant retention. The main study limitation was the open‐label design, which was unavoidable as different injection volumes and injection pens were required for Gla‐300 and Gla‐100.
In conclusion, in Japanese adults with type 1 diabetes using basal and mealtime insulin, there was no between‐treatment difference in HbA1c reduction over 6 months of treatment. Average pre‐injection SMPG was significantly lower with Gla‐300 versus Gla‐100 at month 6. Less hypoglycaemia was observed with Gla‐300, particularly at night, and even in the first 8 weeks of treatment, versus Gla‐100.
Conflict of Interest
M. M. has received research support from Terumo, Nikkiso, Tanabe‐Mitsubishi and Astellas, and has served on speakers bureaus for Sanofi, Novo Nordisk and Novartis. M. K. is an employee and stock/shareholder of Sanofi. X. C. is an employee of Sanofi. Y. T. is an employee and stock/shareholder of Sanofi. M. C. R. has received research support from Amylin, Eli Lilly and Sanofi, and honoraria for consulting and/or speaking from Amylin, Bristol‐Myers Squibb–AstraZeneca Alliance, Elcelyx, Eli Lilly, Hoffmann‐La Roche, Sanofi and Valeritas. These dualities of interest have been reviewed and managed by Oregon Health & Science University. G. B. B. has received honoraria for advising and lecturing from Sanofi, Eli Lilly and Novartis. T. H. has served on an advisory panel for Sanofi, Eli Lilly and Novo Nordisk, has received research support from Sanofi, Eli Lilly, Novo Nordisk, Takeda, Daiichi‐Sankyo, Tanabe‐Mitsubishi, Merck (MSD), Dainippon‐Sumitomo, Novartis, Kissei, Boehringer Ingelheim, Astellas, Terumo, Johnson & Johnson, Ono and Roche, has served on speakers bureaus for Sanofi, Eli Lilly, Novo Nordisk, Takeda, Daiichi‐Sankyo, Tanabe‐Mitsubishi, Merck (MSD), Dainippon‐Sumitomo, Novartis, Kissei, Boehringer Ingelheim, Astellas, Terumo, Johnson & Johnson, Ono and Roche, and has served on the Sanofi insulin‐dosing committee.
Sanofi was the sponsor of the study, and was responsible for the design and coordination of the trial, monitoring clinical sites, collecting and managing data, and performing all statistical analyses. M. M. collected the data and participated in reviewing and editing the manuscript as principal investigator. M. K. contributed to the design of the study protocol and reviewed the manuscript. X. C. conducted the study and reviewed the manuscript as study director. Y. T. participated in writing, reviewing and editing the manuscript. M. C. R. and G. B. B. participated in analysing the findings and writing, reviewing and editing the manuscript. T. H. reviewed the data relating to glycaemic control and dose titration as a member of the Insulin‐Dosing Supervision Committee and reviewed the manuscript.
Supporting information
Appendix S1. Further details of statistical analyses and list of investigators.
Figure S1. Annualized rates of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic events by clock time (safety population).
Figure S2. Change in body weight between baseline and month 6 (safety population).
Table S1. Glycaemic responses over the 6‐month study period (modified intention‐to‐treat population).
Table S2. Number (annualized rates) of hypoglycaemic events for all definitions across the 6‐month study period (safety population).
Table S3. Number (percentage) of participants experiencing ≥1 hypoglycaemic event over 6 months (all definitions; safety population).
Table S4. Most commonly experienced treatment‐emergent adverse events (TEAEs), treatment‐emergent serious adverse events and TEAEs leading to permanent discontinuation during the 6‐month study period (safety population).
Acknowledgements
This study was funded by Sanofi. The authors thank the study participants, trial staff and investigators for their participation. A list of the principal investigators is included in Appendix S1. The authors would also like to thank Cassandra Pessina (Sanofi) for critical review of the manuscript, and for assistance with management of the manuscript development. Editorial assistance was funded by Sanofi and provided by Catriona Marshall of Fishawack Communications. Masayoshi Koyama is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. American Diabetes Association . Standards of medical care in diabetes – 2015. 7. Approaches to glycemic treatment. Diabetes Care 2015; 38: S41–S48. [DOI] [PubMed] [Google Scholar]
- 2. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Japan Diabetes Society . Treatment guide for diabetes 2013; 2012–2013.
- 4. Recent product approvals in Japan. Inpharma Weekly 2003; 1411: 21. [Google Scholar]
- 5. Kanazawa Y, Igarashi Y, Komiya K et al. Long‐term efficacy of insulin glargine after switching from NPH insulin as intensive replacement of basal insulin in Japanese diabetes mellitus. Comparison of efficacy between type 1 and type 2 diabetes (JUN‐LAN study 1.2). Endocr J 2007; 54: 975–983. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto‐Honda R, Takahashi Y, Yoshida Y et al. Use of insulin glargine in Japanese patients with type 1 diabetes. Intern Med 2007; 46: 937–943. [DOI] [PubMed] [Google Scholar]
- 7. Chan JC, Gagliardino JJ, Baik SH et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009; 32: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988‐2010. Diabetes Care 2013; 36: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vouri SM, Shaw RF, Waterbury NV, Egge JA, Alexander B. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm 2011; 17: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolli GB, Andreoli AM, Lucidi P. Optimizing the replacement of basal insulin in type 1 diabetes mellitus: no longer an elusive goal in the post‐NPH era. Diabetes Technol Ther 2011; 13(Suppl. 1): S43–S52. [DOI] [PubMed] [Google Scholar]
- 11. Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: what progress have we made? Diabetes Metab Res Rev 2014; 30: 104–119. [DOI] [PubMed] [Google Scholar]
- 12. Becker RH, Nowotny I, Teichert L, Bergmann K, Kapitza C. Low within‐ and between‐day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab 2015; 17: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker RHA, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units.mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units.mL‐1 . Diabetes Care 2015; 38: 637–643. [DOI] [PubMed] [Google Scholar]
- 14. Shiramoto M, Eto T, Irie S et al. Single‐dose new 0insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab 2015; 17: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bolli GB, Riddle MC, Bergenstal RM et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Home PD, Bergenstal RM, Bolli GB et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care 2015; 38: 2217–2225. [DOI] [PubMed] [Google Scholar]
- 17. Riddle MC, Bolli GB, Zieman M et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using basal and mealtime insulin: glucose and hypoglycemia in a 6‐month randomized controlled trial (EDITION I). Diabetes Care 2014; 37: 2755–2762. [DOI] [PubMed] [Google Scholar]
- 18. Terauchi Y, Koyama M, Cheng X, Shimizu S, Hirose T. Glycemic control and hypoglycaemia in Japanese people with type 2 diabetes mellitus receiving new insulin glargine 300 U/mL in combination with OADs (EDITION JP 2). Diabetologia 2014; 57: S401. [Google Scholar]
- 19. Yki‐Järvinen H, Bergenstal R, Zieman M et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care 2014; 37: 3235–3243. [DOI] [PubMed] [Google Scholar]
- 20. Bergenstal R, Bailey TS, Rodbard D, Guo H, Muehlen‐Bartmer I, Ahmann AJ. Insulin glargine 300 U/mL vs 100 U/mL: glucose profiles of morning vs evening injections in adults with T1DM measured with continuous glucose monitoring (CGM). Diabetes Technol Ther 2015; 17: A16–A17. [Google Scholar]
- 21. ICH . ICH harmonized tripartite guideline: guideline for good clinical practice E6 (R1), 1996. Available from URL: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 19 January 2016. [PubMed]
- 22. World Medical Association . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Ferney‐Voltaire: World Medical Association, 2013. [Google Scholar]
- 23. ADA Workgroup on Hypoglycemia . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 24. Heller S, Buse J, Fisher M et al. Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 25. Mathieu C, Hollander P, Miranda‐Palma B et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26‐week randomized, treat‐to‐target trial with a 26‐week extension. J Clin Endocrinol Metab 2013; 98: 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003; 26: 1902–1912. [DOI] [PubMed] [Google Scholar]
- 27. Gerstein HC, Bosch J, Dagenais GR et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012; 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 28. Iwamoto Y, Clauson P, Nishida T, Kaku K. Insulin degludec in Japanese patients with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Invest 2013; 4: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Further details of statistical analyses and list of investigators.
Figure S1. Annualized rates of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic events by clock time (safety population).
Figure S2. Change in body weight between baseline and month 6 (safety population).
Table S1. Glycaemic responses over the 6‐month study period (modified intention‐to‐treat population).
Table S2. Number (annualized rates) of hypoglycaemic events for all definitions across the 6‐month study period (safety population).
Table S3. Number (percentage) of participants experiencing ≥1 hypoglycaemic event over 6 months (all definitions; safety population).
Table S4. Most commonly experienced treatment‐emergent adverse events (TEAEs), treatment‐emergent serious adverse events and TEAEs leading to permanent discontinuation during the 6‐month study period (safety population).


