Abstract
Introduction
To compare health services utilization and payments for cancer patients who received an implantable intrathecal drug delivery (IDD) system, consisting of a pump and catheter, vs. conventional medical management (CMM) for the treatment of cancer‐related pain.
Methods
This retrospective claims‐data analysis compared health services utilization and payments in a population of patients receiving either IDD or CMM for treatment of cancer pain. Patients were propensity score‐matched 1:1 based on characteristics including, but not limited to, age, gender, cancer type, comorbid conditions, and health care utilization and payments.
Results
From a sample of 142 IDD patients and 3188 CMM patients who met all inclusion/exclusion criteria, 73 matched pairs were obtained. In the year following implant, IDD patients had a consistent trend of lower medical utilization, and total payments that were $3195 lower compared to CMM.
Conclusions
Despite the high initial cost of IDD, this analysis suggests that patients with IDD incur lower medical utilization and payments over the first year post‐implant. Further analysis comprised of a larger, longitudinal sample would contribute to health economics and outcomes research, and assist with future practice guideline development.
Keywords: Cancer pain, conventional medical management, health services utilization, intrathecal drug delivery, retrospective study
Introduction
The financial costs of cancer care are a burden to cancer patients, their families, and society as a whole. National cancer care expenditures have been steadily increasing in the United States and are likely to continue to increase as new, more advanced, and often more expensive treatments are adopted as standards of care. The National Cancer Institute calculated the costs for cancer care in the United States in 2010 to be $125 billion 1. The economic costs of cancer pain are also substantial. In a Swedish study, mean annual costs per patient were 38% higher for patients with cancer pain (€10,400) compared with patients with chronic non‐cancer pain (€6400) 2. Abernethy et al. calculated intervention costs from direct medical resource utilization and unit costs based on a model using a population of 100,000 individuals with demographics similar to the U.S. population 3. The cost for treatment of cancer‐related pain was USD $579 for guideline‐based care, $466 for oncology‐based care, and $315 for usual care, which translates to costs of $118,436, $95,300, and $64,540 per 100,000 population, respectively 3.
The majority of cancer patients experience pain during the course of their disease and treatment, and approximately 25% of cancer patients die in pain 4. Although the number of surviving cancer patients in the United States is increasing and expected to reach 18.1 million by 2020, many cancer survivors have multiple, persistent symptoms, including fatigue, distress, pain, and cognitive impairment, which result in a decreased quality of life 5, 6, 7. Symptoms related to cancer and/or its treatment were reported in 92% of survivors one year after diagnosis 7. More than 25% of cancer survivors have high symptom burden, with pain, fatigue, and depression having the greatest impact on health‐related quality of life (p < 0.0001) 7. Additionally, cancer patients with high levels of pain (>3 on a visual analog scale [VAS] linked to a 0–10 cm numeric scale) have statistically significantly fewer months of survival than cancer patients with low pain levels (≤3 on VAS) (p = 0.002) 8.
Purpose
With U.S. health care reform under way, there is increased scrutiny to demonstrate the most clinically‐effective and cost‐effective ways to treat cancer patients. This is especially relevant in the United States given that nearly 20% of gross domestic product is spent on health care. The goal of this analysis was to use administrative claims data which provide details of real‐world patterns of care for individuals based on claims submitted for payment by health care providers, to compare health services utilization and payments in cancer patients who received an intrathecal drug delivery (IDD) system, consisting of a pump and catheter, vs. conventional medical management (CMM) for the treatment of cancer pain.
Patients and Methods
Data Source
We utilized proprietary U.S. health care information provided in the MarketScan® Commercial Claims and Encounters Database from Truven Health Analytics (Ann Arbor, MI). This data base contains de‐identified, person‐specific insurance enrollment/plan benefit data and health data for fully adjudicated and paid claims, including clinical utilization, expenditures, inpatient care, outpatient care, and outpatient prescriptions. The data base covers 31–45 million individuals annually between 2006 and 2010 and includes private sector health data from approximately 100 payers. These data can be linked to track detailed patient information across sites and types of providers and over time.
Study Population Selection
IDD Patients
Patients with an inpatient or outpatient claim for an IDD system (CPT: 62362) implanted between July 1, 2006 and September 30, 2010 were identified. Patients were also required to have a diagnosis for cancer (ICD‐9‐CM: 140.xx‐209.xx, 230.xx‐234.xx, 235.xx‐239.xx) coded in any position on the pump implant claim. Additionally, a minimum of six months of pharmacy data were required. Finally, patients had to be continuously enrolled in their health plan for a minimum of six months before and two months after their IDD system implant. Patients were excluded if they: 1) did not meet the inclusion criteria or 2) had a history of IDD based on pump programming, analysis, refill, or removal codes (CPT: 95990, 95991, 62367, 62368, 62355, 62365) occurring prior to implant.
CMM Patients
Patients with an inpatient hospital claim including a diagnosis of cancer coded in any position on the claim (ICD‐9‐CM: 140.xx‐209.xx, 230.xx‐234.xx, 235.xx‐239.xx) between July 1, 2006 and September 30, 2010 were identified. The hospital claim also had to include at least two additional diagnosis codes for symptoms associated with uncontrolled cancer pain or chronic opioid use. These included nausea and/or vomiting (ICD‐9‐CM 787.01–787.03), anorexia (783.0), cachexia (799.4), somnolence (780.09), constipation (564.0), fecal impaction (560.32), bowel obstruction (560.9), or decreased mental status (780.97). The patient also had to fill at least one prescription for an opioid in the six months prior to their hospitalization. Finally, patients had to be continuously enrolled in their health plan for a minimum of six months before and two months after their hospitalization. Patients were excluded if they: 1) did not meet the inclusion criteria, or 2) had any evidence of IDD during the entire analysis period (CPT: 62350, 62351, 62361, 62362, 62355, 62365, 62367, 62368, 95990, 95991).
Matching
Patients who receive IDD might be selected for treatment due to patient or physician characteristics that are markedly different from patients receiving CMM. To control for this potential selection bias in claims data, IDD and CMM patients were matched 1:1 based on the Mayo Clinic propensity score matching technique 9. Approximating randomization, the propensity score matching technique provides an unbiased estimate of the effect of treatment by matching patient characteristics. In this case, the technique removed observed differences in the characteristics of patients who received IDD vs. CMM. The propensity score is the predicted probability, ranging from 0 to 1, of belonging to either the IDD group or the CMM group. To obtain the score, a logistic, stepwise regression was conducted to achieve parsimony and best model fit. The p‐value cut‐off for an independent variable's entry into the regression was p = 0.10. The matching logic used a nearest neighbor greedy algorithm, in which the maximum distance between scores for a matched pair was set at 0.01. The groups were compared pre‐ and post‐match using test statistics appropriate to the underlying data distribution (Student's t, chi‐square). A two‐tailed p value = 0.05 was used as a cut‐off for statistical significance. All programming was completed using SAS® 9.2 (Cary, NC).
Statistical Analysis
Timeframe
The first pump claim between 7/1/06 and 9/30/10 was designated as the index date for IDD patients. The first hospitalization between July 1, 2006 and September 30, 2010 was designated as the index date for CMM patients. The claims data in the six‐month period prior to the index date (as early as January 1, 2006) were used to characterize demographics, comorbidities, and other health services utilization and payments. The costs were recorded in U.S. dollars in the years the services were incurred, without adjustments for inflation. The claims data in the period 2, 6, and 12 months following the index date was used to compare utilization and payments after propensity score matching. Survival data were not available in the claims data base. As a result, only patients continuously enrolled at the follow‐up point (2, 6, and 12 months) were included in the analysis for the follow‐up point, respectively. To insure the two groups remained similar despite attrition over the follow‐up period, the patient characteristics in the six months prior to index were retested by group with the remaining sample at 6 and 12 months.
Baseline Period
In the six‐month period prior to index, a series of variables was created a priori for consideration in the matching procedure based on the assumption that they would either be predictive of receiving IDD or predictive of an outcome of interest (e.g., health services utilization or payments). Between‐group differences were compared using descriptive statistics and bivariate tests appropriate to the underlying data distribution. These included: age, gender, region, health plan type, year of index date, baseline utilization, baseline payment, type of cancer, presence of multiple cancers, metastatic cancer, Charlson Comorbidity Index score, treatment by oncologist, treatment by pain specialist, prescription drug use (opioids/tramadol, psychostimulants, laxatives, appetite enhancers, corticosteroids, anticonvulsants, antidepressants, antiemetics, anxiolytics), tumor ablation by approach (i.e., endoscopic, external, open, percutaneous), biopsy by approach, tumor destruction by approach, tumor excision by approach, tumor resection by approach, radiation therapy and chemotherapy.
Follow‐Up Period
Health services utilization and payments for the matched pairs of IDD and CMM patients were compared at 2, 6, and 12 months following the index date. Group differences were compared using descriptive statistics and bivariate tests appropriate to matched samples and to the underlying data distribution (Wilcoxon signed‐rank for nonparametric continuous variables).
Results
Patient Population
IDD Patients
Of the 3625 patients who received their first implanted IDD system between July 1, 2006 and September 30, 2010, 281 (8%) had cancer. Of those, 182 (64.8%) were continuously enrolled in their health plan for a minimum of six months before and two months after their index date. Of the 182 patients, 143 (78.6%) had pharmacy claims data available, as some payers do not submit their pharmacy claims to the MarketScan data base. One patient appearing in both treatment groups was deleted. Therefore, 142 IDD patients comprised the IDD sample before matching.
CMM Patients
There were 13,137 patients hospitalized between July 1, 2006 and September 30, 2010 with a diagnosis code of cancer and at least two additional codes associated with uncontrolled pain or chronic opioid use. Of these, 8381 (63.8%) were continuously enrolled in their health plan for a minimum of six months before and two months after their index date. Of these, 5982 (71.4%) had pharmacy claims data available for the six‐month baseline period. Of these, 32 (0.5%) had evidence of IDD in the six months prior to index and were deleted. Of the remaining 5950 patients, 3188 (53.6%) had at least one opioid prescription at baseline. Therefore, 3188 patients comprised the CMM sample before matching.
Pre‐ and Post‐Match Baseline Characteristics
The propensity score match revealed several patient characteristics that were statistically significantly different between the IDD and CMM groups prior to index, suggesting the patients who received IDD represented a different subset of cancer pain patients compared with patients who received CMM (Table 1). To illustrate, patients with IDD had a higher average number of hospitalizations, more days in hospital, more cases of metastatic disease and multiple cancers, and were more likely to have visited a pain specialist.
Table 1.
Patient Characteristics Six Months Prior to Index: Pre‐ and Post‐Match.
| Characteristic | Pre‐match CMM (N = 3188) | Pre‐match IDD (N = 142) | Pre‐match p value* | Post‐match p value* (n = 73 pairs) | |
|---|---|---|---|---|---|
| Age | Mean | 51.55 | 51.92 | 0.6755 | 0.7552 |
| Emergency room visits† | Mean | 0.96 | 1.42 | 0.01‡ | 0.5266 |
| Inpatient hospital visits† | Mean | 0.25 | 1.13 | <0.0001‡ | 0.9125 |
| Outpatient hospital visits | Mean | 9.36 | 13.55 | <0.0001‡ | 0.1171 |
| Physician office visits | Mean | 14.31 | 18.97 | <0.0001‡ | 0.1739 |
| Days in hospital† | Mean | 1.18 | 7.35 | <0.0001‡ | 0.7954 |
| Total payments† | Mean | 38409.7 | 77393.3 | <0.0001‡ | 0.7711 |
| Home health visits | Mean | 1.07 | 3.62 | <0.0001‡ | 0.0400‡ |
| Hospice days | Mean | 0.04 | 0.43 | 0.0024‡ | 0.1566 |
| Laboratory services | Mean | 1.43 | 1.55 | 0.6416 | 0.0204‡ |
| Gender | |||||
| Males | N | 1168 | 55 | 0.6124 | 0.2038 |
| Females | N | 2020 | 87 | ||
| Year of index date | |||||
| 2006 | N | 302 | 19 | 0.0286‡ | 0.0176‡ |
| 2007 | N | 607 | 32 | ||
| 2008 | N | 741 | 31 | ||
| 2009 | N | 852 | 44 | ||
| 2010† | N | 686 | 16 | ||
| Health plan type | |||||
| Comprehensive | N | 158 | 8 | 0.3265 | 0.5173 |
| Exclusive provider organization | N | 29 | 0 | ||
| Health maintenance organization | N | 546 | 23 | ||
| Place of service | N | 226 | 11 | ||
| Preferred provider organization | N | 1994 | 82 | ||
| Place of service with capitation | N | 16 | 1 | ||
| Consumer‐directed health plan | N | 80 | 5 | ||
| High‐deductible health plan | N | 21 | 3 | ||
| Unknown | N | 118 | 9 | ||
| Region† | |||||
| Northeast | N | 239 | 10 | <0.0001‡ | 0.2989 |
| North Central | N | 892 | 55 | ||
| South | N | 1574 | 39 | ||
| West | N | 476 | 38 | ||
| Unknown | N | 7 | 0 | ||
| Multiple cancers† | |||||
| No | N | 1177 | 12 | <0.0001‡ | 0.7852 |
| Yes | N | 2011 | 130 | ||
| Any diabetes prescription§ | Sum | 380 | 18 | 0.7857 | 0.0706 |
| Antihypertensive use§ | Sum | 1001 | 42 | 0.6470 | 0.4761 |
| Tumor ablation, endoscopic# | Sum | 10 | 2 | 0.0332‡ | 0.5596 |
| Tumor ablation, external | Sum | 0 | 0 | NA | NA |
| Tumor ablation, open | Sum | 0 | 0 | NA | NA |
| Tumor ablation, percutaneous | Sum | 4 | 0 | 0.6728 | 0.3156 |
| Tumor ablation, percutaneous endoscopic | Sum | 0 | 0 | NA | NA |
| Tumor ablation via body | Sum | 2 | 0 | 0.7653 | NA |
| Anticonvulsant use† | Sum | 358 | 44 | <0.0001‡ | 0.1313 |
| Antidepressant use | Sum | 1013 | 64 | 0.0009‡ | 0.0325‡ |
| Antiemetic use† | Sum | 1603 | 83 | 0.0568 | 0.7400 |
| Anxiolytic use | Sum | 1499 | 84 | 0.0046‡ | 0.6162 |
| Biopsy, endoscopic# ,† | Sum | 699 | 17 | 0.0047‡ | 0.6552 |
| Biopsy, external† | Sum | 126 | 2 | 0.1229 | 1.0000 |
| Biopsy, open | Sum | 241 | 11 | 0.9343 | 0.7539 |
| Biopsy, percutaneous† | Sum | 705 | 27 | 0.3828 | 1.0000 |
| Biopsy, percutaneous endoscopic | Sum | 23 | 2 | 0.3534 | 0.3156 |
| Biopsy, via body† | Sum | 126 | 0 | 0.0157‡ | NA |
| Chemotherapy | Sum | 1372 | 89 | <0.0001‡ | 0.8685 |
| Corticosteroid use | Sum | 1111 | 56 | 0.2623 | 0.1668 |
| Tumor destruction, endoscopic# | Sum | 0 | 0 | NA | NA |
| Tumor destruction, open | Sum | 1 | 0 | 0.8328 | NA |
| Tumor destruction, via body | Sum | 0 | 0 | NA | NA |
| Appetite enhancer use | Sum | 754 | 54 | <0.0001‡ | 0.2727 |
| Tumor excision, endoscopic# | Sum | 150 | 5 | 0.5123 | 0.2452 |
| Tumor excision, external | Sum | 44 | 1 | 0.4949 | 0.3156 |
| Tumor excision, open | Sum | 79 | 5 | 0.4380 | 1.0000 |
| Tumor excision, percutaneous | Sum | 8 | 0 | 0.5501 | NA |
| Tumor excision, percutaneous endoscopic | Sum | 0 | 0 | NA | NA |
| Tumor excision, via body | Sum | 1 | 0 | 0.8328 | NA |
| Laxative use | Sum | 226 | 4 | 0.0495‡ | 0.1722 |
| Metastatic disease† | Sum | 1401 | 111 | <0.0001‡ | 0.5747 |
| Treated by oncologist† | Sum | 1089 | 72 | <0.0001‡ | 0.1268 |
| Opioid/tramadol use | Sum | 3188 | 132 | <0.0001‡ | NA |
| Treated by pain specialist† | Sum | 88 | 27 | <0.0001‡ | 1.0000 |
| Psychostimulant use | Sum | 81 | 6 | 0.2182 | 1.0000 |
| Radiation therapy | Sum | 651 | 52 | <0.0001‡ | 0.5528 |
| Tumor resection, open# | Sum | 4 | 0 | 0.6728 | NA |
| Bone cancer† | Sum | 163 | 25 | <0.0001‡ | 0.7714 |
| Breast cancer† | Sum | 568 | 31 | 0.2230 | 0.1748 |
| Carcinoma | Sum | 288 | 15 | 0.5352 | 1.0000 |
| Colorectal cancer | Sum | 436 | 23 | 0.3939 | 0.7658 |
| Digestive system cancer | Sum | 941 | 66 | <0.0001‡ | 0.3548 |
| Endocrine cancer | Sum | 34 | 1 | 0.6787 | 0.6129 |
| Genitourinary cancer† | Sum | 432 | 27 | 0.0646 | 0.3156 |
| Kaposi's sarcoma | Sum | 4 | 1 | 0.0814 | 0.1651 |
| Lung cancer† | Sum | 359 | 38 | <0.0001‡ | 0.4335 |
| Lymphoma/leukemia | Sum | 1300 | 90 | <0.0001‡ | 0.5060 |
| Oral cancer | Sum | 204 | 3 | 0.0385‡ | 1.0000 |
| Other type of cancer | Sum | 1778 | 106 | <0.0001‡ | 0.4706 |
| Pancreatic cancer† | Sum | 173 | 16 | 0.0032‡ | 0.5962 |
| Prostate cancer† | Sum | 99 | 13 | <0.0001‡ | 0.4670 |
| Respiratory/thoracic cancer | Sum | 100 | 3 | 0.4904 | 0.1544 |
| Skin cancer | Sum | 168 | 7 | 0.8589 | 0.7308 |
| Charlson comorbidity Index score components | |||||
| Acute myocardial infarction | Sum | 14 | 0 | 0.4287 | 0.3156 |
| AIDS | Sum | 13 | 0 | 0.4458 | NA |
| Acute ulcer | Sum | 56 | 3 | 0.7530 | 1.0000 |
| Congestive heart failure | Sum | 70 | 2 | 0.5280 | 0.4044 |
| Cirrhosis | Sum | 34 | 1 | 0.6787 | 1.0000 |
| Chronic pulmonary disease | Sum | 386 | 14 | 0.4200 | 0.5740 |
| Chronic ulcer | Sum | 14 | 1 | 0.6444 | 0.3156 |
| Cardiovascular disease | Sum | 113 | 4 | 0.6450 | 0.4670 |
| Dementia | Sum | 4 | 0 | 0.6728 | NA |
| Diabetes† | Sum | 456 | 16 | 0.3101 | 0.7852 |
| Diabetes with sequelae | Sum | 104 | 3 | 0.4472 | 0.1544 |
| Liver disease | Sum | 12 | 0 | 0.4639 | NA |
| Myocardial infarction (history) | Sum | 22 | 1 | 0.9841 | 0.3156 |
| Plegia (all types)† | Sum | 6 | 3 | <0.0001‡ | 0.3156 |
| Peripheral vascular disease | Sum | 46 | 2 | 0.9731 | 0.3156 |
| Renal disease | Sum | 111 | 2 | 0.1818 | 0.3156 |
| Rheumatoid arthritis | Sum | 56 | 0 | 0.1112 | 0.0801 |
Statistical test performed appropriate to the variable type and underlying data distribution.
Variable remained in stepwise logistic regression for developing propensity score at p < 0.10 level of significance.
Indicates a statistically significant difference at the p < 0.05 level.
Due to the short six‐month baseline period, use of drugs for diabetes and hypertension, as two common comorbidities, was included to increase our chances of identification of these conditions above and beyond the inclusion of the Charlson Comorbidity Index score components.
Tumor biopsy, excision, resection, destruction, and ablation were categorized based on the level of invasiveness of the approach.
CMM, conventional medical management; IDD, intrathecal drug delivery.
Propensity Score Match
Similarly, the distribution of propensity scores was markedly different between the two groups prior to index, depicting how the IDD and CMM patients were characteristically unique and emphasizing the importance of employing a methodology to address for potential selection bias. After matching patients 1:1 using their propensity score, 73 matched pairs (n = 146) were retained for further analysis. The average propensity score for the 73 matched IDD patients was 0.1177318. The average propensity score for the 73 matched CMM patients was 0.1177252.
Following the match, with few exceptions, nearly all variables were no longer statistically different between the groups. For those remaining significant differences, the associations were not particularly strong. Therefore, we determined that no further statistical adjustment via regression was required to compare outcomes, and simple bivariate tests were used for post‐index analysis.
Post‐Match Study Population
Patient attrition was expected given the diagnosis of cancer. At two months, 73 matched pairs were continuously enrolled and thus included in the analysis. At six months, 50 IDD patients and 39 CMM patients were included in the analysis. At 12 months, 29 IDD patients and 23 CMM patients were included in the analysis. Re‐testing of baseline characteristics by group at 6 and 12 months revealed no significant differences indicating the groups remained similar and follow‐up comparisons were appropriate.
Post‐Match Follow‐Up Outcomes
Medical Utilization
IDD patients had a significantly lower average number of inpatient hospitalizations (two months, p = 0.0010; six months, p = 0.0358), outpatient hospital visits (six months, p = 0.0133), emergency department visits (two months, p < 0.0001; six months, p = 0.0049), and lab services (six months, p = 0.0168) compared with CMM patients at all follow‐up times with statistical significance achieved at the time points shown (Fig. 1). While the number of physician office visits was fewer at two months in the IDD patients, it was slightly higher at 6 and 12 months. Conversely, while the number of home health and hospice visits was higher at two and six months for IDD patients (hospice at two months, p = 0.0301), it was fewer at 12 months. IDD patients had an average of one day less in hospital after two months, and 4 days after 12 months, compared with CMM patients.
Figure 1.
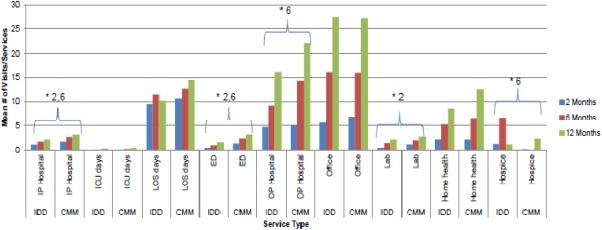
Medical utilization by treatment group over 12 months. * Indicates a statistically significant difference at the p < 0.05 level for the time point(s) listed. No adjustments made for multiple comparisons. CMM, conventional medical management; ED, emergency department; ICU, intensive care unit; IDD, intrathecal drug delivery; IP, inpatient; LOS, length of stay; OP, outpatient.
Pharmacy Utilization
The IDD group had a lower average number of anxiolytic, antiemetic (two months, p = 0.0029; six months, p = 0.0130), corticosteroid (two months, p = 0.0076) and appetite enhancer prescriptions compared with CMM patients at all follow‐up times with statistical significance achieved at the time points shown (Fig. 2). However, IDD patients had a higher average number of antidepressant (two months, p = 0.0136; six months, p = 0.0116), psychostimulant (not significant), and opioid prescriptions (not significant).
Figure 2.
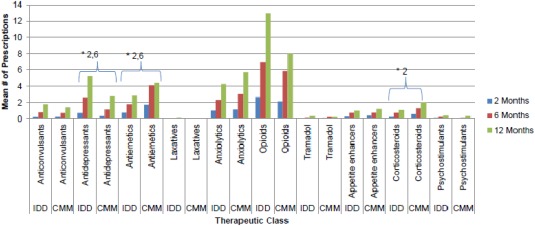
Pharmacy utilization by treatment group over 12 months. * Indicates a statistically significant difference at the p < 0.05 level for the time point(s) listed. No adjustments made for multiple comparisons. CMM, conventional medical management; ED, emergency department; ICU, intensive care unit; IDD, intrathecal drug delivery; IP, inpatient; LOS, length of stay; OP, outpatient.
Payments
IDD patients had lower emergency department (two months, p < 0.0001; 12 months, p = 0.0076), home health, and lab payments compared with CMM patients at all follow‐up times with statistical significance achieved at time points shown. Although not significantly different, pharmacy payments were consistently higher compared with CMM patients (Table 2; Fig. 3). Payments for inpatient hospitalizations, outpatient hospital visits, and hospice care started out higher for IDD patients, yet were all lower by 12 months (hospice at six months, p = 0.0113). Conversely, physician office payments started out lower for IDD patients compared with CMM patients and were higher by 12 months (two months, p = 0.0160). Combined medical and total payments (medical + pharmacy + cost of IDD implant) were higher for IDD patients at two months ($58,209 vs. $55,157 for CMM), but lower after six months compared with CMM patients ($97,761 vs. $103,306, respectively; Fig. 4). At 12 months, pharmacy costs were $9264 higher for IDD while medical costs were $12,459 lower, achieving a total cost savings of $3195 for IDD relative to CMM. When the costs associated with the index date for both groups were excluded (i.e., cost of IDD system, cost of hospitalization for CMM), the costs associated with IDD were $7255 lower than with CMM.
Table 2.
Payments by Treatment Group Over 12 Months (U.S. Dollars).
| Payment type | Month | N | Intrathecal drug delivery (IDD) | Conventional medical management (CMM) | Mean difference IDD‐CMM | t‐test p value* | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard deviation | Mean | Median | Standard deviation | |||||
| Inpatient hospital | 2 | 73/73 | $36,779 | $10,479 | $104,790 | $35,033 | $23,136 | $38,332 | $1746 | 0.8938 |
| 6 | 50/39 | $47,692 | $15,614 | $128,009 | $41,251 | $28,163 | $39,985 | $6441 | 0.7629 | |
| 12 | 29/23 | $40,218 | $23,372 | $48,300 | $44,341 | $34,321 | $34,420 | ($4123) | 0.7312 | |
| Emergency department | 2 | 73/73 | $300 | $0 | $660 | $1152 | $622 | $1550 | ($852) | <0.0001* |
| 6 | 50/39 | $664 | $0 | $1684 | $1511 | $732 | $2371 | ($847) | 0.0521 | |
| 12 | 29/23 | $584 | $148 | $1133 | $2486 | $1416 | $3466 | ($1902) | 0.0076* | |
| Outpatient hospital | 2 | 73/73 | $13,163 | $6772 | $15,194 | $8884 | $2124 | $13,527 | $4279 | 0.0744 |
| 6 | 50/39 | $24,499 | $16,091 | $28,616 | $30,097 | $10,981 | $40,856 | ($5598) | 0.4497 | |
| 12 | 29/23 | $41,037 | $28,911 | $43,574 | $47,181 | $14,493 | $67,531 | ($6144) | 0.6929 | |
| Physician office | 2 | 73/73 | $3726 | $1184 | $6722 | $7260 | $1549 | $10,407 | ($3534) | 0.0160* |
| 6 | 50/39 | $13,829 | $4770 | $20,491 | $21,164 | $2149 | $31,501 | ($7335) | 0.1881 | |
| 12 | 29/23 | $27,004 | $5040 | $38,662 | $25,050 | $3664 | $45,984 | $1954 | 0.8685 | |
| Lab | 2 | 73/73 | $77 | $0 | $450 | $139 | $0 | $528 | ($62) | 0.4473 |
| 6 | 50/39 | $122 | $0 | $542 | $124 | $0 | $294 | ($2) | 0.9892 | |
| 12 | 29/23 | $112 | $0 | $420 | $123 | $0 | $290 | ($11) | 0.9192 | |
| Home health | 2 | 73/73 | $605 | $0 | $1374 | $715 | $0 | $1899 | ($110) | 0.6868 |
| 6 | 50/39 | $1784 | $508 | $5781 | $4701 | $0 | $19,815 | ($2917) | 0.3250 | |
| 12 | 29/23 | $3092 | $507 | $9243 | $4182 | $587 | $9967 | ($1090) | 0.6850 | |
| Hospice | 2 | 73/73 | $926 | $0 | $3982 | $232 | $0 | $1259 | $694 | 0.1573 |
| 6 | 50/39 | $2179 | $0 | $5222 | $12 | $0 | $76 | $2167 | 0.0113* | |
| 12 | 29/23 | $376 | $0 | $1388 | $1121 | $0 | $3587 | ($745) | 0.3089 | |
| Other misc. | 2 | 73/73 | $770 | $0 | $3397 | $434 | $0 | $1833 | $336 | 0.4588 |
| 6 | 50/39 | $1092 | $0 | $6014 | $344 | $0 | $763 | $748 | 0.4424 | |
| 12 | 29/23 | $315 | $0 | $768 | $713 | $0 | $1353 | ($398) | 0.1870 | |
| Medical subtotal | 2 | 73/73 | $56,346 | $34,481 | $106,518 | $53,849 | $43,907 | $38,530 | $2497 | 0.8509 |
| 6 | 50/39 | $91,863 | $63,900 | $129,831 | $99,204 | $84,203 | $71,314 | ($7341) | 0.7516 | |
| 12 | 29/23 | $112,739 | $91,331 | $91,548 | $125,198 | $119,840 | $73,165 | ($12,459) | 0.5974 | |
| Pharmacy | 2 | 73/73 | $1863 | $889 | $2401 | $1308 | $611 | $1767 | $555 | 0.1138 |
| 6 | 50/39 | $5898 | $2537 | $9296 | $4102 | $1875 | $7955 | $1796 | 0.3385 | |
| 12 | 29/23 | $13,668 | $4116 | $24,232 | $4404 | $2551 | $5791 | $9264 | 0.0795 | |
| Total | 2 | 73/73 | $58,209 | $35,370 | $55,157 | $44,518 | $3052 | 0.8181 | ||
| 6 | 50/39 | $97,761 | $66,437 | $103,306 | $86,078 | ($5545) | 0.8107 | |||
| 12 | 29/23 | $126,407 | $95,447 | $129,602 | $122,391 | ($3195) | 0.8979 | |||
Indicates a statistically significant difference at the p < 0.05 level for the time point(s) listed. No adjustments made for multiple comparisons.
Figure 3.
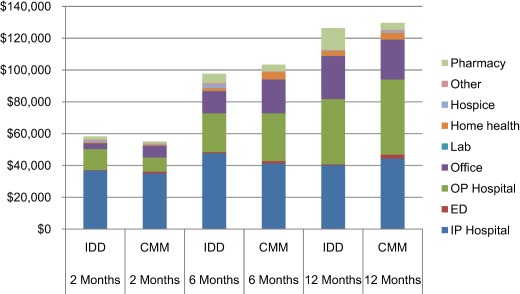
Payments by treatment group over 12 months (U.S. dollars). CMM, conventional medical management; ED, emergency department; IDD, intrathecal drug delivery; IP, inpatient; OP, outpatient.
Figure 4.
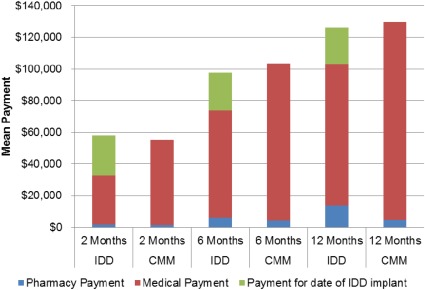
Contribution of IDD implant to total payments (U.S. dollars). The mean difference in total payments favored CMM at two months ($55,157 for CMM vs. $58,209 for IDD), IDD at six months ($97,761 for IDD vs. $103,306 for CMM), and IDD at 12 months ($126,407 IDD vs. $129,602 for CMM). CMM, conventional medical management; ED, emergency department; IDD, intrathecal drug delivery; IP, inpatient; OP, outpatient.
Discussion
Recent practice guidelines have emphasized concern over the socioeconomic costs of potential complications and anxieties associated with screening and subsequent interventions. Most prominently, this has involved prostate‐specific antigen screening and therapeutic alternatives, such as watchful waiting, in subgroups of prostate cancer patients, and low‐dose CT screening for lung cancer in at‐risk patients. Such preventive efforts are increasingly being scrutinized on the basis of cost as well as efficacy. Indeed, in May 2014 the Medicare Evidence & Coverage Advisory Committee recommended against coverage for annual lung cancer screening in at‐risk persons, although screening had been endorsed by more than 40 medical societies and a surgical cure of stage 1 lung cancer is often possible 10. With the increased influence of health care economics and outcomes research, futile care at the end of life, which consumes 25% of the entire Medicare budget, is also a target for cost cutting. The budget sequester included a 2% across‐the board reduction in Medicare spending in 2013, and during the past five years health care spending in the U.S. has grown at historically low rates (3.6–4.1% annually) 11. Further cuts are inevitable. Advocacy of a costly intervention to palliate cancer pain, often at the end of life, must therefore be justified on the basis of ethics to relieve suffering, and efficacy that is superior to CMM.
The cost of IDD vs. CMM for cancer pain management depends, as is shown in this study, on the length of observed follow‐up where, in this analysis, continuous enrollment was used as a proxy for survival. Thus, patient selection is critical in order for interventional cancer pain management to be cost effective, and is highly dependent on physician judgment of prognostic factors that determine length of survival. We were cognizant that the number of IDD recipients in the cancer pain population would be small, and that sample size would drop over time due, in part, to length of survival.
The literature on the use of IDD in cancer pain includes a selection of case series, best practices consensus guidelines, and two randomized controlled trials 4, 12, 13, 14, 15. In the randomized trial by Smith and colleagues, IDD patients had statistically significantly lower toxicity scores (50% reduction) compared to patients receiving conventional treatment (e.g., systemic opioids) for pain management (17% reduction, p = 0.004). IDD also lowered pain scores by 52% vs. 39% in the CMM group (p = 0.055) 14.
While the existing studies focus on the clinical and quality‐of‐life benefits of IDD, it is important to explore whether IDD provides economic benefits, in terms of lower direct costs to payers, or potential indirect cost benefits, such as fewer readmissions or fewer days in hospital for patients. Consequently, this retrospective claims analysis compared health services utilization and payments, from a payer perspective, in patients with cancer receiving either IDD or CMM for the management of cancer‐related pain.
The use of propensity score matching was deemed appropriate to address potential selection bias, as IDD patients might be systematically different from CMM patients. Indeed, the distribution of characteristics prior to index and propensity scores demonstrated that across multiple dimensions the two patient groups were disparate. For example, IDD patients had more metastatic disease, were more likely to visit a pain specialist, and were more likely to have a hospitalization and longer length of stay during the baseline period. After propensity score matching, however, the two groups were very similar with scores within 0.01 for a pair of characteristics. The use of simple bivariate statistics to test associations was reasonable, given that the few variables that remained significant after matching were unlikely to affect key outcomes including hospitalization, length of stay, and total medical costs.
Over the 12‐month follow‐up, IDD patients had lower medical utilization and lower total medical payments, driven by the savings in hospitals and emergency departments. The nonsignificant differences might have been affected by lack of power to detect a difference given the small sample. However, IDD patients had higher use of certain classes of medications, such as opioids and antidepressants, and total pharmacy payments were higher compared with CMM patients.
Limitations
Administrative claims data bases are developed for the purpose of payment. Moreover, utilization and payments are only reflective of what was coded, with the assumption that most coding is accurate and complete. Additionally, claims data cannot capture over‐the‐counter utilization of medications or adherence to therapy. For the cancer population specifically, claims data do not include measures of severity, such as tumor staging or quality‐of‐life information. Given the advanced clinical condition for many cancer pain patients, especially those who receive IDD, we observed a measurable decline in enrollment status post‐index. We were unable to discern whether patients no longer were enrolled in their health plan or died, unless they died in hospital. The small sample size likely affected our ability to detect statistically significant differences between treatment groups despite apparent trends. In terms of the analytical approach, the use of propensity score matching can only control for observed differences between treatment groups; unknown or unobserved confounding may still exist. While the use of instrumental variables could have addressed this limitation, the identification of an appropriate instrumental variable for this patient population is challenging. Finally, these findings are only generalizable to other U.S. commercially insured populations and not to Medicare or Medicaid patients.
Conclusion
Despite the high initial cost of IDD, this retrospective analysis of cancer pain patients suggests that patients with IDD incur lower medical utilization and payments over the first year post‐implant compared with CMM. Total average payments for IDD were $3195 lower per patient than for CMM. Future research with a larger, longitudinal sample and with patient‐reported outcomes, including pain severity and quality of life, would contribute to health economics and outcomes research, and assist with future guideline development.
Authorship Statements
Dr. Stearns, Ms. Hammond, and Mr. Berryman contributed to the study conception and design, manuscript writing, and final manuscript approval. Ms. Hinnenthal contributed to the study conception and design, data collection, analysis and interpretation, manuscript writing, and final manuscript approval. Dr. Janjan contributed to data analysis and interpretation, manuscript writing, and final manuscript approval.
Acknowledgement
We would like to acknowledge Tina Hunter, PhD, Executive Director of Outcomes Research at S2 Statistical Solutions, Inc., for her contribution to consulting on the statistical analysis plan and performing a portion of the testing.
Conflict of Interest: Dr. Stearns is a consultant for Medtronic, Inc. and Medallion Therapeutics, and has received research support from the Alfred Mann Foundation, Mallinckrodt, GW Pharma, Medallion Therapeutics, and Medtronic, Inc. Dr. Janjan received honoraria from CMP Healthcare Publishers, Oncology, and The ASCO Post, and is consultant for CommGeniX. Dr. Stearns is an owner/employee of the Center for Pain and Supportive Care. Ms. Hinnenthal is an employee of Medtronic, Inc. Ms. Hinnenthal owns stock in Medtronic, Inc. Ms. Hammond is an employee of the Center for Pain and Supportive Care. Mr. Berryman is an employee of Hospital Corporation of America.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This research was supported by Medtronic, Inc.
References
- 1. National Cancer Institute at the National Institutes of Health . The cost of cancer. Bethesda, MD: National Institutes of Health, 2012. [Google Scholar]
- 2. Gustavsson A, Bjorkman J, Ljungcrantz C et al. Socio‐economic burden of patients with a diagnosis related to chronic pain—register data of 840,000 Swedish patients. Eur J Pain 2012;16:289–299. [DOI] [PubMed] [Google Scholar]
- 3. Abernethy AP, Samsa G, Matchar DB. A clinical decision and economic analysis model of cancer pain management. Am J Manag Care 2003;9:651–664. [PubMed] [Google Scholar]
- 4. Stearns L, Boortz‐Marx R, Du Pen S et al. Intrathecal drug delivery for the management of cancer pain: a multidisciplinary consensus of best clinical practices. J Support Oncol 2005;3:399–408. [PubMed] [Google Scholar]
- 5. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burkett VS, Cleeland CS. Symptom burden in cancer survivorship. J Cancer Surviv 2007;1:167–175. [DOI] [PubMed] [Google Scholar]
- 7. Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Study of Cancer Survivors. Cancer 2011;117:2779–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boström B, Sandh M, Lundberg D, Fridlund B. A comparison of pain and health‐related quality of life between two groups of cancer patients with differing average levels of pain. J Clin Nurs 2003;12:726–735. [DOI] [PubMed] [Google Scholar]
- 9. Kosanke J, Bergstralh E. Locally written SAS macros. Rochester, MN: Mayo Clinic Division of Biomedical Statistics and Informatics, 2012. [Google Scholar]
- 10. Center for Medicare & Medicaid Services . 2014. Proposed decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG‐00439N). http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=274. Accessed January 18, 2014.
- 11. Hartman M, Martin AB, Lassman D, Catlin A; National Health Expenditure Accounts Team . National health spending in 2013: growth slows, remains in step with the overall economy. Health Affairs 2015;34:150–160. [DOI] [PubMed] [Google Scholar]
- 12. Gilmer‐Hill HS, Boggan JE, Smith KA, Frey CF, Wagner FC Jr, Hein LJ. Intrathecal morphine delivered via subcutaneous pump for intractable pain in pancreatic cancer. Surg Neurol 1999;51:6–11. [DOI] [PubMed] [Google Scholar]
- 13. Hassenbusch SJ, Pillay PK, Magdinec M et al. Constant infusion of morphine for intractable cancer pain using an implanted pump. J Neurosurg 1990;73:405–409. [DOI] [PubMed] [Google Scholar]
- 14. Smith TJ, Staats PS, Deer T et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug‐related toxicity, and survival. J Clin Oncol 2002;20:4040–4049. [DOI] [PubMed] [Google Scholar]
- 15. Staats PS, Yearwood T, Charapata SG et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA 2004;291:63–70. [DOI] [PubMed] [Google Scholar]


