Figure 1.
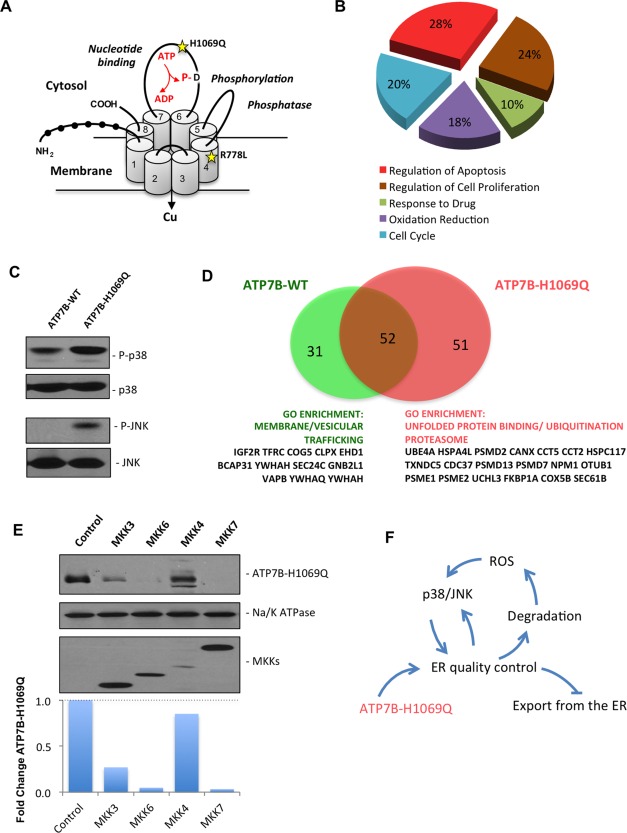
Expression of the ATP7BH1069Q mutant is associated with activation of p38 and JNK signaling pathways. (A) Schematic structure of ATP7B. Black circles show N‐terminal metal binding domains. Numbers indicate transmembrane helices. The domains which regulate adenosine triphosphatase activity are indicated in italic with D residue for catalytic phosphorylation. Yellow stars indicate the position of the most frequent WD‐causing mutations, H1069Q and R778L. (B) HepG2 cells were infected with Ad‐ATP7BWT‐GFP or Ad‐ATP7BH1069Q‐GFP and prepared for microarray analysis (see Materials and Methods). Genes that were differently expressed in cells expressing ATP7BH1069Q were analyzed for GO enrichment. The pie diagram shows the GO categories that were enriched among the altered genes in ATP7BH1069Q‐expressing cells, as opposed to cells expressing ATP7BWT (see also Supporting Table S1). Genes involved in the regulation of apoptosis constituted the largest group of genes whose expression was altered by the ATP7BH1069Q mutant. (C) HepG2 cells were infected with Ad‐ATP7BWT‐GFP or Ad‐ATP7BH1069Q‐GFP and analyzed with western blot. Phosphorylated forms of p38 or JNK increased in cells expressing the ATP7BH1069Q mutant, while overall amounts of p38 or JNK remained similar in wild type‐expressing and mutant‐expressing cells. (D) Putative interactors of ATP7BWT and ATP7BH1069Q were identified using a proteomics approach (see Materials and Methods). The diagram shows the number of interactors that were specific for ATP7BWT or for ATP7BH1069Q, as well as the number of common interactors. GO analysis revealed ATP7BWT interactors to be enriched in proteins belonging to membrane trafficking categories, while mutant‐specific interactors were enriched in proteins involved in ER‐associated protein quality control and degradation. (E) HepG2 cells expressing ATP7BH1069Q were transfected with activators of p38 (MKK3 and MKK6) or JNK (MKK4 and MKK7). Western blot (see also quantification graph) revealed a decrease in ATP7BH1069Q levels in cells expressing p38 or JNK activators. Na/K‐adenosine triphosphatase was used as input control. The modest decrease in ATP7BH1069Q in cells transfected with MKK4 is due to lower overexpression of MKK4 in comparison to other MKKs. (F) The schematic drawing shows a vicious circle that is generated by expression of the ATP7BH1069Q mutant, which leads to activation of ER quality control and degradation of ATP7BH1069Q. As a consequence of ATP7BH1069Q loss, ROS increase and stimulate p38 and JNK signaling, which triggers further retention and degradation of the mutant in the ER, preventing it from being exported to the secretory pathway. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; P‐, phosphorylated.
