Figure 2.
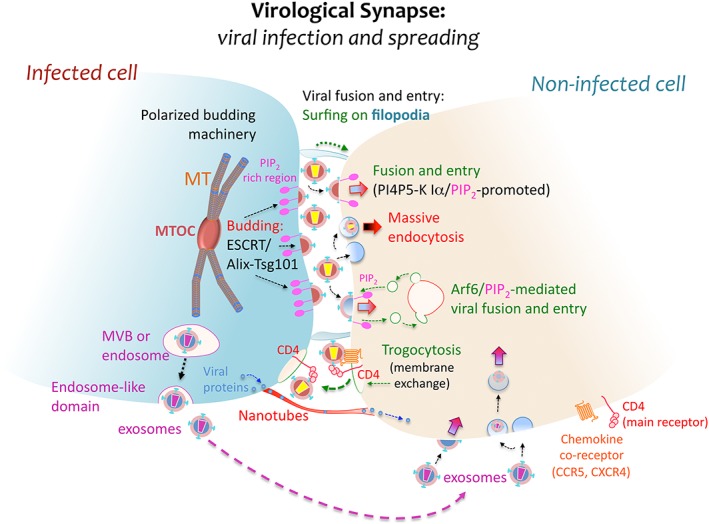
Virological synapse and spreading. At the virological synapse (VS), some viruses attach structural polyproteins to PIP2‐rich membrane regions of the infected cell for further budding and release into the intercellular space. PIP2 confers fluidity to the cell membrane and favours virus–cell fusion. These virions then bind to specific receptors in order to infect the neighbouring target cell at the VS, fusing with its plasma membrane directly or after surfing on actin‐structured filopodia, or being internalized by endocytosis as is believed to occur with HIV‐1. The VS represents an efficient environment for viral budding. It typically arises in PIP2‐enriched plasma membrane domains, where the membrane of the infected cell is polarized towards the synaptic junction through the movement of vesicles governed by the ESCRT/Alix‐Tsg101 machinery or by MVBs coordinating the translocation of the MTOC. This scaffolding facilitates subsequent viral infection and spread from the infected to the nearby uninfected cell. In addition, long membrane nanotubes may also form between neighbouring cells, promoting viral protein trafficking. Other dynamic membrane events involved in viral infection and spreading are trogocytosis, Arf6/PIP2‐mediated membrane dynamics and exosomal transport. Trogocytosis involves the exchange of cell surface membrane patches that may contain receptor clusters associated to viral particles, while exosomes are vesicles formed from MVBs that could participate in viral infection and spreading between cells
