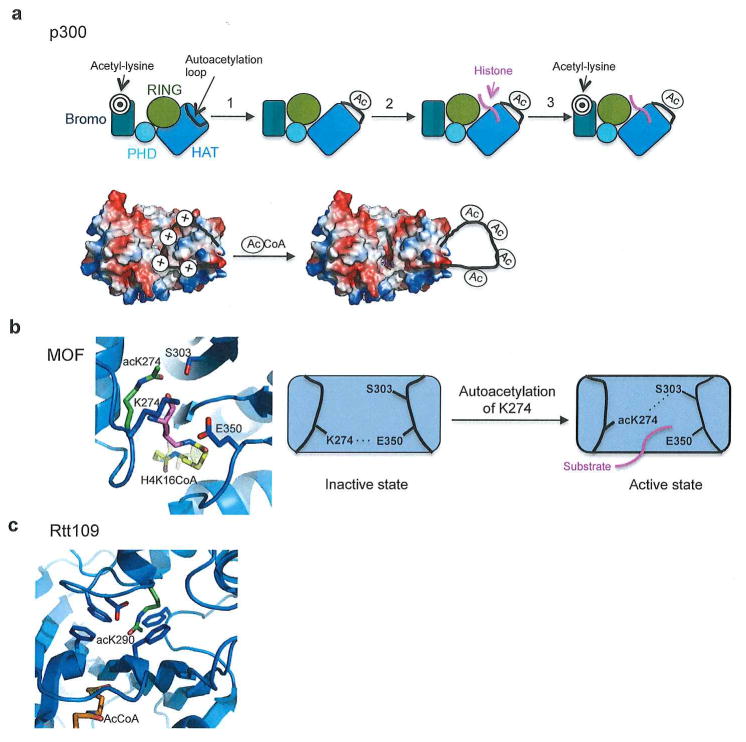
Figure 5. HAT Schematics.
(a) Top- Schematic of proposed p300 mechanism. HAT domain is colored marine, RING domain is colored gold, PHD domain is colored cyan, Bromodomain is colored dark teal, and substrate binding loop is colored black, correlating with Figure 1. It is possible that p300 uses its bromodomain initially to recruit target substrates (represented by target symbol). Step 1: p300 becomes acetylated at the autoinhibitory loop that displaces the loop and the RING domain. Step 2: p300 is able to acetylate substrates (shown in pink). Step 3: Bromodomain domain may bind target substrates following initial substrate acetylation to regulate further acetylation. Figure adapted from44. Bottom- Model for p300 activation by autoacetylation. Figure adapted from70. (b) Close up and schematic of autoacetylation of MYST family acetyltransferases (MOF used as a representative member.) When unacetylated K274 forms a hydrogen bond with the general base Glu350. When K274 becomes acetylated (shown in green) it flips 90° out of the active site and forms a hydrogen bond with Ser303, which may permit substrate binding (shown in pink). Overlaying of the H4K16CoA from the ESA1/H4K16CoA structure (PDB code: 3TO6) reveals that the substrate would clash with unacetylated K274. Figure adapted from70. (C) The autoacetylated K290 (shown in green) of Rtt109 in its buried environment. Figure adapted from70.