Figure 1.
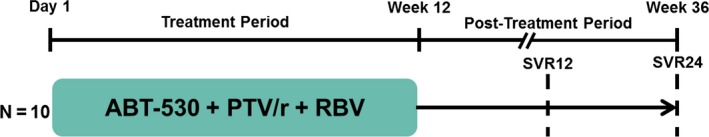
Study design. This study consisted of a 12‐week treatment period followed by a 24‐week post‐treatment period. PTV/r, paritaprevir/ritonavir; RBV, ribavirin; SVR12, sustained virological response at post‐treatment week 12 (primary endpoint); SVR24, sustained virological response at post‐treatment week 24.
