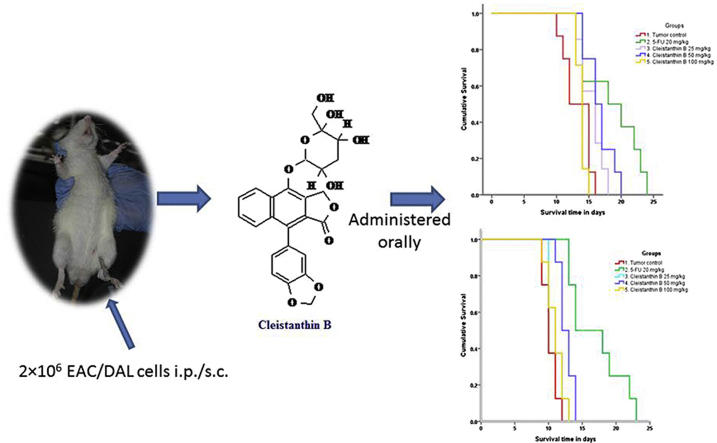
Abstract
To evaluate the in vivo antitumor activity of cleistanthin B in Ehrlich's ascites carcinoma (EAC) and Dalton's ascites lymphoma (DAL) cell lines induced malignant ascites mouse models and DAL cell line induced solid tumor mouse model. All animals were injected with 2 × 106 EAC/DAL cells i.p./s.c. to induce malignant ascites and solid tumor and treated with 5-fluorouracil (5-FU) 20 mg/kg or cleistanthin B for 10 days. Cleistanthin B was given at three doses viz. 25, 50 and 100 mg/kg. The percentage increase in life span and the overall survival in malignant ascites animals and the tumor volume in solid tumor animals were measured. The haematological parameters were assessed in all animals before and 2 weeks after the treatment. Cleistanthin B 50 mg/kg and 5-FU significantly prolonged the life span (>25%) of malignant ascites tumor bearing animals. The overall survival was significantly improved by both. Only cleistanthin B 50 mg/kg significantly reduced the elevated WBC counts in EAC tumor bearing animals. Both 5-FU and cleistanthin B 50 mg/kg reversed the malignancy induced increase in neutrophils and platelet counts and decrease in lymphocyte counts but not to the normal range. Only 5-FU significantly reduced the solid tumor volume. None of the three doses of cleistanthin B was effective against the solid tumor. Cleistanthin B has antitumor activity against EAC and DAL tumor mice but it is not as effective as 5-FU. At 50 mg/kg dose cleistanthin B exerts significant antitumor activity compared to 25 and 100 mg/kg dose. Its effect on WBC count is higher and advantageous when compared to 5-FU. But cleistanthin B in the doses used is not effective against solid tumor.
Keywords: Cleistanthin B, Dalton's ascites lymphoma (DAL), Ehrlich's ascites carcinoma (EAC), 5-Fluorouracil, Solid tumor
Graphical abstract
1. Introduction
Cancer is one of the leading causes of death worldwide and the burden is increasing day by day. Though we have a number of anticancer agents now, adequate control of cancer is still lacking. Hence there is a persistent demand to develop newer and more effective anticancer drugs which can help tackle this problem. The major groups of anticancer drugs such as vinca alkaloids, taxanes, camptothecins and epipodophyllotoxins which are currently a part of many standard anticancer regimens are derived from plants.1 Cleistanthin B is an arylnaphthalene lignan lactones glycosides, the major phytoconstituents responsible for most of the toxicological and pharmacological actions of Cleistanthus collinus, a plant commonly found in the states of southern India.2 Apart from cleistanthin B, plant has cleistanthin A, collinusin, and diphyllin.3
Cleistanthin B also present in Dysosma versipellis (Berberidaceae), a Chinese herb widely used to clear sputum, kill parasites and treat epidemic encephalitis B and epidemic parotitis,4 and Hypoestes purpurea (Acanthaceae), a tropical herb eaten as spinach, used as a poultice for sore eyes.5, 6 Cleistanthins A and B were reported to possess hypotensive7 and diuretic8 properties. While these compounds have no activity on motor function,9 they were demonstrated to exhibit significant antitumor activity.10, 11, 12, 13, 14 Most of the studies about antitumor activity of these compounds are based on in vitro assays. The GI50 (50% growth inhibition) values are lower for tumor cells compared to normal cells.11, 13 They exert cytotoxic activity on various cancer cell lines by interfering with cell cycle progression, causing DNA damage and inducing apoptosis.10 While cleistanthin A has in vivo antitumor activity against Dalton's ascites lymphoma (DAL) and S-180 sarcoma tumor bearing animals by prolonging the life span and reducing the tumor volume,11 there is no report of the in vivo antitumor activity of cleistanthin B so far. Hence the present study was focused to evaluate the in vivo antitumor activity of cleistanthin B in Ehrlich's ascites carcinoma (EAC) and DAL cell line induced malignant ascites and solid tumor in mice.
2. Materials and methods
2.1. Test compound
Cleistanthin B was isolated from the leaves of C. collinus (Euphorbiaceae) as described in our previous study.15 Taxonomically identified plant leaves were collected in the regions of Pondicherry district and it was certified by Botanical survey of India, Coimbatore (BSI/SC/5/23/08-09/Tech.241).
2.2. Cancer cell lines
EAC and DAL cell lines (Amala Cancer Research Center, Thrissur, Kerala) were maintained by weekly intraperitoneal transplantation of respective tumor cells (2 × 106 cells per mouse) in stock animals.
2.3. Chemicals
Carboxymethyl cellulose sodium (CMC) (Lobachemie, Mumbai), phosphate buffered saline (PBS) (Media labs, Mumbai), Diethyl ether (Thermo Fisher Scientific India Pvt Ltd, Mumbai) and 5-fluorouracil (5-FU) (Ceon labs, Andhra Pradesh) were used for the study.
2.4. Instruments
Automated haematology analyzer (SYSMEX XS-1000i; Sysmex Corporation, Kobe, Japan) was used for the complete blood cell counts.
2.5. Animals
Female Swiss albino mice were obtained from the central animal house of JIPMER, Pondicherry and they were maintained under standard laboratory conditions throughout the study. The animals were fed with standard rodent pellet feed (Amrut feeds, Sangli, India) and water ad libitum. Adult mice weighing 20–30 g were used for the experiments. The study protocol was approved by the Institutional Animal Ethics Committee (No: Jip/Micro/Jiaec/2012) and all the animal experiments were carried out in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
2.6. Study design
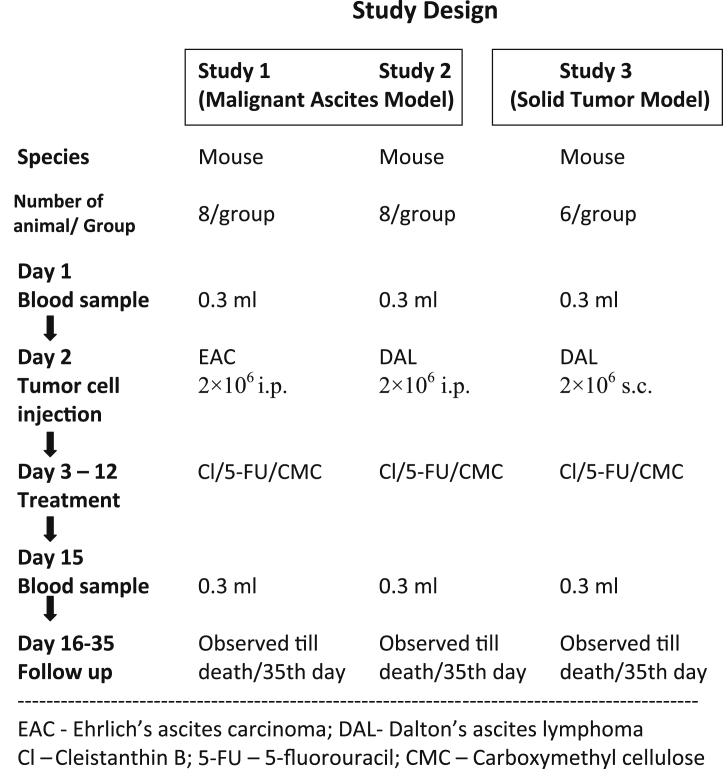
An exploratory study was designed to evaluate the in vivo antitumor activity of cleistanthin B using different mouse tumor models. Study I and II were carried out with EAC and DAL cell lines induced malignant ascites mouse models, respectively. DAL cell line induced solid tumor mouse model was used for study III. Three doses of cleistanthin B viz. 25, 50 and 100 mg/kg were chosen based on the results of a toxicity study done previously.16 The animals were divided into five different groups as follows:
Group I: Tumor control
Group II: 5-FU 20 mg/kg
Group III: Cleistanthin B 25 mg/kg
Group IV: Cleistanthin B 50 mg/kg
Group V: Cleistanthin B 100 mg/kg
Study I and II used 8 mice in each group while study III used 6 mice per group. Group I served as a positive (tumor) control (0.5% CMC orally) and group II as a comparator control (5-fluorouracil 20 mg/kg; i.p.). Groups III, IV and V were given cleistanthin B orally at the doses of 25, 50 and 100 mg/kg respectively. The study design is summarized in Fig. 1.
Fig. 1.
Study plan.
2.7. Experimental procedure
On day 1, blood collection from retro-orbital plexus was carried out and the samples (0.3 ml) in EDTA were used for the assessment of haematological parameters such as haemoglobin (Hb) content, red blood cell (RBC) count, total white blood cell (WBC) count, neutrophils (%), lymphocytes (%) and platelet count. On day 2, tumor fluid was withdrawn from the stock animals for EAC and DAL cell lines and the tumor cell count was done using Neubauer chamber under the light microscope. The PBS was added to make a concentration of 1 × 106 cells in 0.1 ml. For tumor induction in study I and II, each experimental animal was injected with 2 × 106 DAL/EAC cells i.e. 0.2 ml intraperitoneally. In study III, the solid tumor was induced by injecting s.c. injection of 2 × 106 DAL cells in 0.1 ml PBS on right hind limb of each experimental animal. After 24 h of the tumor cells inoculation, the animals were treated according to their respective groups once daily for next 10 days. On day 15, the retro-orbital blood collection was done again for haematological assessment, if the animal was alive.17 The animals were followed till death or upto 35 days. The parameters for antitumor activity in study I and II were recorded as follows:
Determination of the percentage increase in life span (PILS): It is calculated from the mean survival time (MST) values.18 The MST for each group was calculated as,
For each group,
Determination of the overall survival: It was based on the results of survival analysis. In study – III, the antitumor activity was assessed by the reduction of solid tumor volume.
Determination of the solid tumor volume: The solid tumor volume was measured on the seventh day after tumor inoculation and then it was repeated every fifth day until the end of the study. The tumor volume was calculated using the following formula19
where, r1 and r2 are two perpendicular radii of the solid tumor measured using a vernier caliper.
The haematological parameters of all surviving animals such as haemoglobin, RBC, WBC, neutrophils, lymphocytes and platelets were assessed for all the three studies. A group of six normal mice was studied for assessing their haematological parameters. These normal (control) values were used for comparisons. The tumor bearing animals alive at the end of the study were sacrificed by cervical dislocation.
2.8. Statistical analysis
The survival analysis was done by Log-rank test using SPSS version 20 software package (IBM SPSS, USA). The mean survival time, haematological parameters and tumor volume comparison between groups were analyzed using Instat version 3.06 (GraphPad, USA). The statistical tests used were one-way ANOVA and Kruskal–Wallis test. P < 0.05 was considered statistically significant.
3. Results
3.1. The antitumor activity of cleistanthin B against malignant ascites tumor bearing animals
3.1.1. Effect on the survival
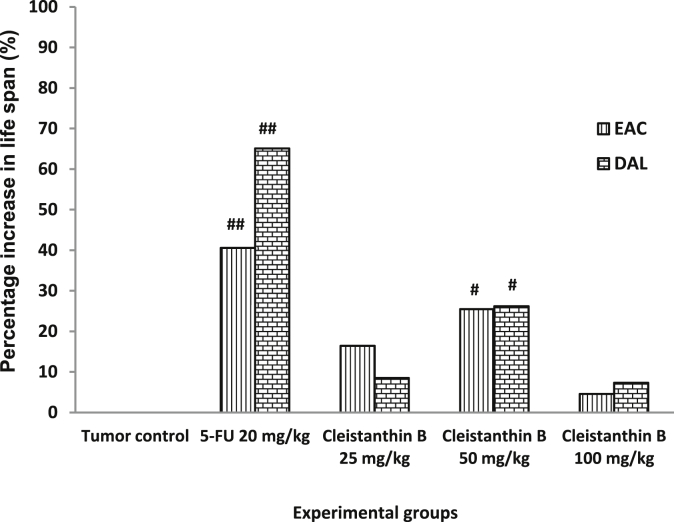
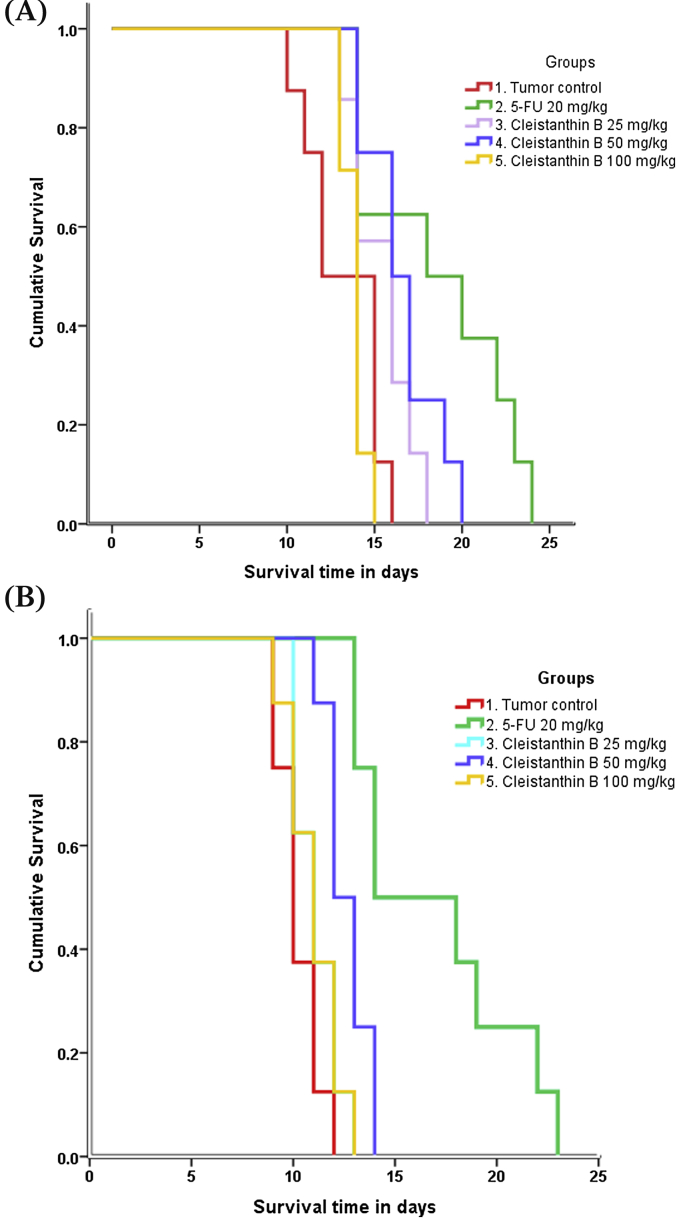
Cleistanthin B 50 mg/kg and 5-FU significantly increased the PILS. While 5-FU increased the life span of EAC and DAL animals by 40.6% and 65.1% respectively, cleistanthin B increased it by 25.5% and 26.2% respectively (Fig. 2). Only cleistanthin B 50 mg/kg and 5-FU significantly improved the overall survival of EAC and DAL tumor bearing animals. While cleistanthin B 50 mg/kg and 5-FU were not significantly different from each other in improving the overall survival of EAC animals, 5-FU was significantly more effective than all the three doses of cleistanthin B in improving the overall survival of DAL animals (Fig. 3A and B).
Fig. 2.
Effect of cleistanthin B on percentage increase in life span of Ehrlich's ascites carcinoma (EAC) and Dalton's ascites lymphoma (DAL)tumor bearing mice (n = 8 in each group, #Significant increase (>25%) in PILS compared to tumor control).
Fig. 3.
Effect of cleistanthin B on the overall survival of EAC (A) and DAL (B) tumor bearing mice (n = 8 in each group). #P < 0.01, ##P < 0.001 compared to tumor control. $P < 0.05, $$P < 0.01,$$$P < 0.001 compared to 5-FU.
3.1.2. Effect on the haematological parameters
The haematological parameters could not be analyzed in DAL animals because only four out of 40 animals were alive on day 15. All four animals were in 5-FU group. So mortality was less in 5-FU group compared to all the three doses of cleistanthin B. The Hb and RBC count were lower in all the treatment groups compared to normal control group. The WBC counts were significantly increased in tumor control, 5-FU and cleistanthin B 25 mg/kg group compared to normal control group. Only cleistanthin B 50 mg/kg decreased the WBC counts to normal range. The neutrophils were increased and lymphocytes were decreased significantly in all the treatment groups. The platelet count was significantly increased in tumor control and cleistanthin B 25 mg/kg group compared to normal group (Table 1).
Table 1.
Effect of cleistanthin B on haematological parameters of EAC tumor bearing mice on day 15.
| Group | Treatment | Hb (g/dl) | RBC (1 × 106/μl) | WBC (1 × 103/μl) | Neutrophils (%) | Lymphocytes (%) | Platelets (1 × 103/μl) |
|---|---|---|---|---|---|---|---|
| 0 | Normal control | 14.0 ± 0.23 | 9.27 ± 0.25 | 6.20 ± 0.48 | 13.3 ± 1.41 | 88.2 ± 1.14 | 683.3 ± 32.18 |
| I | Tumor control | 9.5 ± 1.44 | 6.39 ± 1.02 | 75.73 ± 25.17* | 83.5 ± 3.75* | 13.0 ± 3.58** | 1128.0 ± 77.30** |
| II | 5-FU 20 mg/kg | 8.7 ± 1.26* | 5.05 ± 0.71** | 56.92 ± 12.21** | 88.4 ± 2.11** | 10.6 ± 1.78*** | 794.8 ± 51.83 |
| III | Cleistanthin B 25 mg/kg | 8.1 ± 0.10* | 5.42 ± 0.71* | 34.01 ± 4.26* | 78.8 ± 2.87* | 21.3 ± 2.87* | 955.0 ± 74.53* |
| IV | Cleistanthin B 50 mg/kg | 9.3 ± 0.46* | 6.20 ± 0.30 | 11.77 ± 2.78#$ | 67.8 ± 7.35* | 31.8 ± 7.28* | 835.7 ± 20.54 |
Hb: Hemoglobin; RBC: Red Blood Cells; WBC: Wight Blood Cells, 5-FU: 5-Fluorouracil.
The values are expressed as mean ± SEM; n = 6 in group 0 and IV, n = 5 in group II, n = 4 in group I and III.
*P < 0.05, **P < 0.01, ***P < 0.001 compared to normal control; #P < 0.05 compared to tumor control; $P < 0.05 compared to 5-FU.
The data for cleistanthin B 100 mg/kg group is not included because only one animal was alive on day15.
3.2. The antitumor activity of cleistanthin B against solid tumor bearing animals
All animals with solid tumor survived beyond 35 days.
3.2.1. Effect on the tumor volume
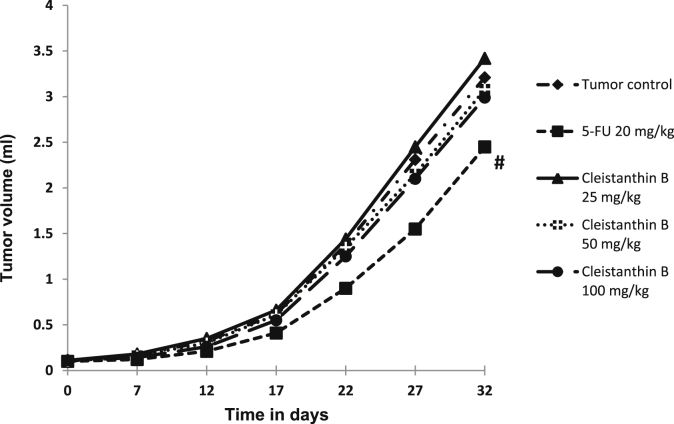
The tumor volume was found to be significantly less only in 5-FU group compared to tumor control group. None of the three doses of cleistanthin B significantly reduced the solid tumor volume (Fig. 4).
Fig. 4.
: Effect of cleistanthin B on the tumor volume of DAL solid tumor bearing mice. n = 6 in each group; #P < 0.001 compared to tumor control, when area under the curves were compared between groups.
3.2.2. Effect on the haematological parameters
There was no significant difference between the groups with regards to haematological parameters (Data not shown).
4. Discussion
In the present study, cleistanthin B showed significant antitumor activity against EAC and DAL cell line induced malignant ascites tumor animals at 50 mg/kg dose while no significant antitumor activity was recorded against DAL solid tumor animals with all the three doses of cleistanthin B. Even the higher dose of cleistanthin B was found to be less effective than 5-FU which was used as a standard comparator.
The life span was prolonged by cleistanthin B more than 25% in both EAC and DAL tumor animals. Prolongation of life span is a valuable criterion for initial evaluation of antitumor activity of a new compound.20, 21 If the compound increases the life span by 25%, it can be considered to have a significant antitumor activity.22 The results of survival analysis should also be considered for assessing the antitumor activity and cleistanthin B has improved the overall survival of animals. There is no previous report on the in vivo antitumor activity of cleistanthin B but in vitro studies with cleistanthins A and B have reported the same. The antineoplastic activity was predicted in silico for both the compounds by PASS bioinformatics tool2 and cleistanthin A which has been evaluated in vivo in DAL tumor animals was reported to have significant antitumor activity.11 Since cleistanthins A and B are structurally similar, it is not surprising that cleistanthin B shares the same in vivo effect.
While cleistanthin B 50 mg/kg significantly decreased the WBC count in EAC tumor animals, 5-FU could not do so. The agents with antitumor activity tend to decrease the WBC count and it is a reliable criterion of their antineoplastic activity.23 The increase in WBC count in tumor bearing animals was probably due to myeloid leukemoid reaction secondary to malignancy. The significant increase in WBC count may be a defensive mechanism towards tumor cells. The antitumor effect of a compound will reduce the tumor cells and decrease the WBC count. The reduction in leucocyte count probably reflected the decrease in tumor burden as well as the action of compound on the bone marrow precursors. Palanisamy et al. reported that fishes exposed to C. collinus extract developed leucopenia.24 The haematological parameters in solid tumor animals were not deranged. Although 5-FU showed a marginal decrease in the WBC count, it was not significant while cleistanthin B maintained a near normal range. So it can be said that 5-FU reduces the WBC count when it is raised due to malignancy and also when it is in normal range. In contrast, cleistanthin B reduces WBC count only when it is raised due to malignancy and not when it is in normal state which is advantageous.
The haemoglobin and RBC values were less in all treatment groups. It is well known that development of anemia is a problem with most of the cancer chemotherapy agents. In the present study also we found the same problem with 5-FU and cleistanthin B. The most common cause of anemia in cancer is chemotherapeutic agent induced myelosuppression. The anemia in ascites carcinoma bearing mice can also occur due to iron deficiency or haemolytic conditions. It is known that neutrophils and platelet counts increase while lymphocytes decrease in all the ascites tumor bearing animals.25 Reactive neutrophilic leucocytosis is known in malignancy and the agents with antitumor activity tend to reverse it. Both 5-FU and cleistanthin B reversed the deranged neutrophils (%), lymphocytes (%) and platelet counts but not to the normal range.
Cleistanthin B did not significantly reduce the tumor volume in solid tumor animals. It was reported that cleistanthin B has affinity towards the localized solid tumor and it may be helpful in the treatment of solid tumor.26 But in the current study, we did not observe the beneficial effect of cleistanthin B in solid tumor animals. Cleistanthin B doses were chosen based on the results of a previously carried out toxicological study in animals.16, 27 It is possible that the doses selected could have been inadequate against solid tumor. This is evidenced by the fact that the tumor volume starts increasing rapidly after stopping the treatment. So cleistanthin B given at a higher dose for longer duration might have been effective against solid tumor.
Out of all the three doses of cleistanthin B only 50 mg/kg has shown a significant antitumor activity in malignant ascites tumor bearing animals. While cleistanthin B 25 mg/kg dose may be ineffective because it did not significantly improve the survival of animals, cleistanthin B 100 mg/kg might have exerted toxic effect on the EAC and DAL tumor bearing animals because most of the animals in this group died early.
5. Limitations
Parameters such as viability testing of tumor cells and percentage cell growth inhibition were not evaluated in our study. Early death of ascites tumor animals did not allow us to collect blood on day 15. Reducing the initial load of tumor cells to 1 × 106 cells during inoculation might have helped to circumvent the problem. We have not carried out the tumor growth delay assay in solid tumor model which could be done by measuring the tumor volume every day. These limitations have not affected the outcome of the study but eliminating them would have given more information.
6. Conclusions
From the present study it can be concluded that cleistanthin B at the dose of 50 mg/kg has significant antitumor activity against EAC and DAL ascites tumor bearing mice but it is not as effective as 5-FU. Cleistanthin B has a better effect on WBC count compared to 5-FU but it has no antitumor activity against solid tumor.
Conflict of interest
Nil.
Acknowledgement
The authors thank the administration of JIPMER, Pondicherry for financial support (Grant sanction no. 7(1)2010) and Amala Cancer Research Center, Thrissur, Kerala, India for providing cancer cell lines.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Mahdi A.A., Ansari J.A., Khan H.J. Anticancerous medicinal plants: a review. Int J Adv Pharm Res. 2013;4:1706–1722. [Google Scholar]
- 2.Parasuraman S., Raveendran R. Computer-aided prediction of biological activity spectra, pharmacological and toxicological properties of cleistanthin A and B. Int J Res Pharm Sci. 2010;1:333–337. [Google Scholar]
- 3.Das S., Hamide A., Mohanty M.K., Muthusamy R. Fatal Cleistanthus collinus toxicity: a case report and review of literature. J Forensic Sci. 2014;59(5):1441–1447. doi: 10.1111/1556-4029.12519. [DOI] [PubMed] [Google Scholar]
- 4.Xu X., Gao X., Jin L. Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Div. 2011;6(1):14. doi: 10.1186/1747-1028-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J., Xie G., Yan X. vol. 1. Springer-Verlag Berlin Heidelberg; Berlin: 2011. Encyclopedia of Traditional Chinese Medicines; p. 455. (Isolated Compound A-B). [Google Scholar]
- 6.Hypoestes aristata. Available in http://www.plantzafrica.com/planthij/hypoesaristata.htm [Last accessed 15.07.15].
- 7.Parasuraman S., Raveendran R., Selvaraj R.J. Effects of cleistanthins A and B on blood pressure and electrocardiogram in Wistar rats. Z Für Naturforschung C J Biosci. 2011;66:581–587. doi: 10.1515/znc-2011-11-1207. [DOI] [PubMed] [Google Scholar]
- 8.Parasuraman S., Raveendran R. Diuretic effects of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinusin Wistar rats. J Young Pharm. 2012;4:73–77. doi: 10.4103/0975-1483.96616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhavrao C., Parasuraman S., Raveendran R. Insignificant effect of cleistanthin A and cleistanthin B on motor function in animal models. J Pharm Negat Results. 2013;4:66–70. [Google Scholar]
- 10.Rajkumar S., Bhatia A.L., Shanmugam G. Molecular mechanisms underlying the inhibition of cell proliferation by cleistanthins. Asian J Exp Sci. 2001;15:9–16. [Google Scholar]
- 11.Pradheepkumar C.P., Shanmugam G. Anticancer potential of cleistanthin A isolated from the tropical plant Cleistanthus collinus. Oncol Res. 1999;11:225–232. [PubMed] [Google Scholar]
- 12.Pradheepkumar C.P., Panneerselvam N., Shanmugam G. Cleistanthin A causes DNA strand breaks and induces apoptosis in cultured cells. Mutat Res. 2000;464:185–193. doi: 10.1016/s1383-5718(99)00179-5. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran C., Kumar P., Panneerselvam N., Rajesh S., Shanmugam G. Cytotoxic and genotoxic effects of cleistanthin B in normal and tumour cells. Mutagenesis. 1996;11:553–557. doi: 10.1093/mutage/11.6.553. [DOI] [PubMed] [Google Scholar]
- 14.Kumar C.P., Pande G., Shanmugam G. Cleistanthin B causes G1 arrest and induces apoptosis in mammalian cells. Apoptosis. 1998;3:413–419. doi: 10.1023/a:1009658518998. [DOI] [PubMed] [Google Scholar]
- 15.Parasuraman S., Raveendran R., Vijayakumar B., Velmurugan D., Balamurugan S. Molecular docking and ex vivo pharmacological evaluation of constituents of the leaves of Cleistanthus collinus (Roxb.) (Euphorbiaceae) Indian J Pharmacol. 2012;44:197–203. doi: 10.4103/0253-7613.93848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parasuraman S., Raveendran R., Rajesh N.G., Nandhakumar S. Sub-chronic toxicological evaluation of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus (Roxb.) Toxicol Rep. 2014;1:596–611. doi: 10.1016/j.toxrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1:87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raihan M.O., Tareq S.M., Brishti A., Alam M.K., Haque A., Ali M.S. Evaluation of antitumor activity of Leea indica (Burm.f.) Merr. extract against Ehrlich ascites carcinoma (EAC) bearing Mice. Am J Biomed Sci. 2012;4:143–152. [Google Scholar]
- 19.Loganayaki N., Manian S. Antitumor activity of the methanolic extract of Ammannia baccifera L. against Dalton's ascites lymphoma induced ascitic and solid tumors in mice. J Ethnopharmacol. 2012;142:305–309. doi: 10.1016/j.jep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Fouda F.M. Anti-tumor activity of tetrodotoxin extracted from the Masked Puffer fish Arothron diadematus. Egypt J Biol. 2005;7:1–13. [Google Scholar]
- 21.Muthuraman M.S., Dorairaj S., Rangarajan P., Pemaiah B. Antitumor and antioxidant potential of Tragia Plukenetii R. Smith on Ehrlich ascites carcinoma in mice. Afr J Biotechnol. 2008;7:3527–3530. [Google Scholar]
- 22.Andreani A., Scapini G., Galatulas I., Bossa R. Potential antitumor agents IX: synthesis and antitumor activity of two analogues of ketocaine. J Pharm Sci. 1983;72:814–815. doi: 10.1002/jps.2600720724. [DOI] [PubMed] [Google Scholar]
- 23.Saroja M., Annapoorani S. Antitumor activity of methanolic extract of Terminalia catappa leaves against Ehrlich ascites induced carcinoma in mice. Int Res J Pharm. 2011;2:253–254. [Google Scholar]
- 24.Palanisamy P., Sasikala G., Mallikaraj D., Bhuvaneshwari N., Natarajan G.M. Haematological changes of fresh water food fish, Channa striata on exposure to Cleistanthus collinus suicidal plant extract. Res J Pharm Biol Chem Sci. 2011;2:812–816. [Google Scholar]
- 25.Aranjani J.M., Manuel A., Mallikarjuna Rao C. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of Xanthium strumarium in transplantable tumors in mice. Am J Chin Med. 2013;41:145–162. doi: 10.1142/S0192415X13500110. [DOI] [PubMed] [Google Scholar]
- 26.Parasuraman S., Raveendran R., Ardestani M.S. Biodistribution properties of cleistanthin A and cleistanthin B using magnetic resonance imaging in a normal and tumoric animal model. Pharmacogn Mag. 2012;8:129–134. doi: 10.4103/0973-1296.96559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parasuraman S. JIPMER; Pondicherry: 2011. Isolation of Cleistanthin A and B from Cleistanthus collinus Roxb. (Euphorbiaceae) Leaves and Evaluation of Pharmacological and Toxicological Profile of Cleistanthin A and B in Animal Models. [dissertation] [Google Scholar]