Abstract
Objectives:
Early-life intelligence has been shown to predict multiple causes of death in populations around the world. This finding suggests that intelligence might influence mortality through its effects on a general process of physiological deterioration (i.e., individual variation in “biological age”). We examined whether intelligence could predict measures of aging at midlife before the onset of most age-related disease.
Methods:
We tested whether intelligence assessed in early childhood, middle childhood, and midlife predicted midlife biological age in members of the Dunedin Study, a population-representative birth cohort.
Results:
Lower intelligence predicted more advanced biological age at midlife as captured by perceived facial age, a 10-biomarker algorithm based on data from the National Health and Nutrition Examination Survey (NHANES), and Framingham heart age (r = 0.1–0.2). Correlations between intelligence and telomere length were less consistent. The associations between intelligence and biological age were not explained by differences in childhood health or parental socioeconomic status, and intelligence remained a significant predictor of biological age even when intelligence was assessed before Study members began their formal schooling.
Discussion:
These results suggest that accelerated aging may serve as one of the factors linking low early-life intelligence to increased rates of morbidity and mortality.
Keywords: Aging—Biomarkers, Cognition, IQ, Intelligence
Intelligence in early adulthood and middle age is an important risk factor for early death, predicting risk of premature mortality better than many other commonly assessed risk factors, including blood pressure, dyslipidemia, and body mass index (Batty, Shipley, Gale, Mortensen, & Deary, 2008). A recent meta-analysis of 16 independent studies concluded that a 1 SD advantage in intelligence test scores assessed within the first two decades of life is associated with a 24% lower risk of death over a follow-up period of 17–69 years (Calvin et al., 2011). This body of work forms the backbone of “cognitive epidemiology,” a new field which seeks to document and explain the ways in which intellectual differences influence health and longevity (Deary, 2010). Among the key developments in this field are findings that low intelligence is associated not just with premature death, but also with a range of health conditions, beginning with obesity and the metabolic syndrome in the first half of the life course, followed by type 2 diabetes and heart disease in later life, and dementia in old age (Arden, Gottfredson, & Miller, 2009; Batty et al., 2008; Belsky et al., 2013; Der, Batty, & Deary, 2009; Wrulich et al., 2013).
The challenge for cognitive epidemiology now is to identify why low childhood intelligence is associated with such a diverse array of negative health outcomes. One possibility is that associations between intelligence, disease, and mortality arise because less intelligent people actually “age” faster than their more intelligent peers. The concept of accelerated aging arises from observations that age-related chronic diseases are preceded by a gradual accumulation of damage to multiple organ systems that begins in the first half of the life course (Ben-Shlomo & Kuh, 2002). Consequently, if children with lower intelligence are aging faster, evidence of this acceleration should be detectable even before the onset of chronic diseases that ultimately cause death.
One way to observe accelerated aging before the onset of disease is to examine measures of “biological age.” Measures of biological age capture the progressive deterioration in physiological functioning that transforms the physical and cognitive fitness of healthy adulthood into frailty characterized by increasing vulnerability to injury, disease, and death (Butler et al., 2004). Examples of such measures include specific biomarkers such as leukocyte telomere length (LTL), as well as composite indices that synthesize information from multiple biomarkers, like the Framingham heart age. Importantly, these measures can be taken at any chronological age, and can therefore help to identify individuals who are aging more rapidly than their peers even at younger ages before pathology presents.
We tested the hypothesis that low intelligence predisposes to accelerated aging using four measures of biological age: perceived facial age, a 10-biomarker algorithm developed using data from the National Health and Nutritional Examination Survey (NHANES III; Levine, 2013), an estimate of cardiovascular disease (CVD) risk translated into a measure of “vascular age” using data from the Framingham group (D’Agostino et al., 2008), and LTL. We examined data from the Dunedin Study of a complete birth cohort. The Dunedin Study measured intelligence beginning in early childhood, when cohort members were 3 years old. Biological age was assessed at midlife, when cohort members were aged 38 years—before the onset of most age-related disease.
Whereas associations between certain components (e.g., lung function, C-reactive protein) of our two composite measures of biological age have been explored in relation to intelligence in previous studies (Batty, Deary, Schoon, & Gale, 2007; Calvin, Batty, Lowe, & Deary, 2011; Richards, Strachan, Hardy, Kuh, & Wadsworth, 2005), we chose to examine these markers as constituents of larger composites (where appropriate) because doing so allows for capture of the concurrent age-related decline of multiple biomarkers across a variety of bodily systems (the sign of advancing biological age) as well as more accurate prediction of mortality (D’Agostino et al., 2008; Levine, 2013). In addition, our composite measures are less susceptible to “noise” generated by transient fluctuations in individual markers due to temporary illness or stochastic variation, and minimize the influence of non-error sources of variation seen in specific markers while aggregating the common variance cutting across markers, further enhancing construct validity.
To strengthen the inference that low intelligence contributes to accelerated aging, we also tested whether the association between intelligence and biological age could be accounted for by early-life exposures known to decrease intelligence as well as increase the risk of ill health and disease. For example, preterm birth and low birth weight are risk factors for low IQ (Newcombe, Milne, Caspi, Poulton, & Moffitt, 2007), age-related diseases (Barker, Osmond, Golding, Kuh, & Wadsworth, 1989), and early mortality (D’Onofrio et al., 2013). Thus, infants who suffer more perinatal problems may later display both reduced intelligence and accelerated aging, creating the false impression of a causal relationship. Similarly, childhood illness may interfere with a child’s performance on cognitive tests as well as influence later measures of aging. We therefore included statistical adjustments for perinatal complications and childhood ill health to address these possibilities.
Research designs aimed at untangling socioeconomic status (SES) and intelligence suggest that physical health appears to be more closely associated with intellectual ability than socioeconomic privilege, at least in adolescence (Lubinski & Humphreys, 1992). However, previous research also suggests that children’s early SES influences their intelligence (Von Stumm & Plomin, 2015), and that socioeconomically advantaged children may benefit from resources that promote healthy aging (Strand et al., 2010). To control for a possible confounding effect of some Study members’ early economic privilege, we thus included an additional statistical adjustment for childhood SES.
Finally, education is also likely to affect intelligence test scores (Brinch & Galloway, 2012). However, because the effects of intelligence and educational attainment are reciprocal over the life course, it is difficult to disentangle their effects in observational studies. Consequently, instead of using a statistical covariate to control for educational attainment, we exploited our prospective design to examine whether biological age could be predicted by intelligence tested prior to the start of Study members’ formal schooling. This exceptionally early measure of intelligence provides us with a significant advantage over previous research, which has typically assessed intelligence in early adolescence or young adulthood (Calvin et al., 2011).
Methods
Sample
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (N = 1,037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand who were eligible for the longitudinal study based on residence in the province at age 3, and who participated in the first follow-up assessment at age 3. The cohort represents the full range of SES in the general population of New Zealand’s South Island and is primarily white. Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1,007 Study Members still alive took part. At each assessment wave, each Study member is brought to the Dunedin research unit for a full day of interviews and examinations. There were 30 deaths in the cohort between assessment waves at ages 3 and 38; however, in each case the cause of death was not due to age-related disease. By age 38, only 11 Study members had been diagnosed with an age-related condition such as type II diabetes, myocardial infarction, or stroke. The Otago Ethics Committee approved each phase of the Study and informed consent was obtained from all Study members.
Measures of Intelligence
Intellectual assessments were conducted in early childhood (ages 3 and 5), middle childhood (ages 7, 9, and 11), and again at midlife (age 38). Correlations among our 3 measures of intelligence ranged from 0.577 (early childhood and midlife) to 0.791 (middle childhood and midlife).
Early-childhood intelligence
At age 3, we measured intelligence using two measures of verbal comprehension: the Peabody Picture Vocabulary Test (PPVT; Dunn, 1965) and the Receptive Language Scale from the Reynell Developmental Language Scales (RDLS; Reynell, 1969). On the PPVT, the child is asked to point to one of four pictures in response to a stimulus word; in this way, a measure of verbal comprehension is made. On the RDLS, verbal comprehension is assessed by presenting the child with toys and asking him or her to respond to questions. At age 5, we measured participants’ intelligence using the Stanford–Binet Intelligences Scales (Terman & Merrill, 1960), which involve a variety of tasks set out in age levels from age two to superior adult level centering largely on language comprehension and expression. We then averaged standardized versions of Study members’ ages 3 and 5 intelligence test scores to create a single measure of intelligence in early childhood.
Middle-childhood intelligence
At ages 7, 9, and 11, we report results from the Wechsler Intelligence Scale for Children—Revised (WISC-R; Wechsler, 1974), using participants’ total scores averaged over the three assessment points to represent intelligence in middle-to-late childhood.
Midlife intelligence
At age 38, we report results from the Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV; Wechsler, 2008).
Midlife Aging Outcomes
We used clinical biomarkers alongside other sources of information to create four measures of age 38 biological age. Physical examinations were conducted during the age 38 assessment day at the Dunedin Study Research Unit, with 4-hour postprandial blood draws between 4:15 and 4:45 pm. Table 1 shows the correlations among these four outcome measures.
Table 1.
Correlations Between Age 38 Aging Outcomes
| Perceived facial age | NHANES biomarker algorithm | Framingham heart age | Telomere length | |
|---|---|---|---|---|
| Perceived facial age | 1 | |||
| 956 | ||||
| NHANES algorithm | 0.197*** | 1 | ||
| 904 | 904 | |||
| Framingham heart age | 0.217*** | 0.530*** | 1 | |
| 900 | 900 | 900 | ||
| Telomere length | −0.076* | −0.059 | −0.061 | 1 |
| 829 | 822 | 820 | 829 |
Note: N for each correlation in italics. NHANES = National Health and Nutrition Examination Survey (III).
*p < .05. ** p < .01. *** p < .001.
Perceived facial age
Perceived facial age is an assessment of how old a person appears relative to his or her chronological age, reflecting tissue integrity. We chose to include this measure in our analyses because perceived age is widely used as a general indicator of health by clinicians, and is correlated with early mortality and telomere length (Christensen et al., 2009). Because there is no consensus regarding which approach is the best measure of perceived age, we used two methods. First, age range was assessed by a panel of four undergraduate raters blind to Study members’ actual ages. Raters were presented with standardized (non-smiling) facial photographs of Study members divided into sex-segregated slideshow batches containing approximately 50 photos, viewed for 10s each. Raters were randomized to viewing the slideshow batches in either forward progression or backwards progression and used a Likert scale to categorize each Study member into a 5-year age range (i.e., from 20–24 years old up to 65–70 years). Scores for each Study member were averaged across all raters (α = 0.71; range: 25–29 to 53–57). The second measure, relative age, was assessed by a different panel of four undergraduates. These raters were told that all photos were of people aged 38 years old. Raters then used a 7-item Likert scale to assign a “relative age” to each Study member (1 = “young looking”, 7 = “old looking”). Scores for each Study member were averaged across all raters (α = 0.72; range: 2–6). Because age range and relative age were highly correlated (r = 0.73), we standardized and averaged both variables to create a composite measure of perceived age at 38 years (N = 956).
Biomarker algorithm
Calculating human biological age is a relatively recent enterprise and there is disagreement about methods (Mitnitski & Rockwood, 2013). Our goal was to borrow and implement the most validated approaches. Recently, Levine (2013) used data from a nationally representative, cross-sectional sample of adults aged 30–75 years (NHANES III) to compare the ability of five Biological Age algorithms to predict mortality. Results showed that Klemera and Doubal (2006) method performed the best (i.e., it predicted mortality, did so significantly better than chronological age, and accounted for the association between chronological age and mortality). We chose to include this measure in our analyses because it predicts mortality better than any single biomarker considered in isolation.
This equation takes information from m number of regression lines of chronological age regressed on m number of biomarkers, where x is the value of biomarker j measured for an individual in the Dunedin cohort. For each biomarker j, the parameters k, q, and s BA are estimated from a regression of chronological age on the biomarker using data from NHANES III. Parameters k, q, and s BA, represent the regression intercept, slope, and root mean squared error, respectively, from the age and biomarker-specific regression models. CA represents chronological age (38 for all Dunedin cohort members). Biomarkers used to calculate biological age in the Dunedin cohort are the same as those used in Levine’s (2013) original analysis. (Levine analyzed a panel of 21 biomarkers in the NHANES III sample and included the 10 that were significantly correlated with chronological age at r > 0.1 in the biomarker algorithm.) The biomarkers are: C-reactive protein, glycated hemoglobin, total cholesterol, forced expiratory volume, systolic blood pressure, serum creatinine, serum albumin, serum urea nitrogen, serum alkaline phosphatase, and cytomegalovirus optical density. We excluded Study members who did not consent to phlebotomy or were pregnant at the time of assessment, leaving us with data from 904 Study members (Biomarker algorithm age range = 28.33–61.01 years).
Framingham heart age
Heart age is an estimate of vascular age based on the Framingham CVD risk score, a single multivariable function that predicts risk of developing all CVD and its constituents. We chose to include Framingham heart age in our analyses because the score is commonly used by physicians to communicate cardiovascular disease risk to their patients. The 10-year CVD risk for each Study member was computed using sex-specific factors collected at the age 38 assessment phase including: total cholesterol, HDL cholesterol, systolic blood pressure, treatment for hypertension, diabetes status, and smoking status. Framingham CVD risk was then translated to Heart age using the Heart-age calculators made available by the Framingham group (D’Agostino et al., 2008). We excluded Study members who did not consent to phlebotomy, were pregnant at the time of assessment, or were missing any of the individual variables, leaving us with data from 900 Study members (Framingham heart age range = 22–85 years).
Mean relative LTL
Telomeres, the protective caps at the end of chromosomes, gradually erode in somatic tissues with each division of the cell. We chose to include this measure in our analyses because both animal and human studies show a link between telomere length and early mortality (Deelen et al., 2014), and because telomere erosion can be observed in midlife when most people are still healthy, leading some to liken telomere length to a “biological clock” that captures cellular aging across the lifespan (Lopez-Otin, Blasco, Partridge, Serrano, & Kroemer, 2013). Leukocyte DNA was extracted from the blood of non-Maori ancestry Study members at age 38 using standard procedures (for cultural reasons, DNA from Study members of Maori ancestry are not transported to the United States for analysis). Study members’ DNA was stored at −80°C until assayed to prevent degradation of the samples. LTL was measured using a validated quantitative PCR method, as previously described, which determines mean telomere length across all chromosomes for all cells sampled (Shalev et al., 2014). This method involves two quantitative PCRs for each subject, one for a single-copy gene (S) and the other in the telomeric repeat region (T). All DNA samples were run in triplicate for telomere and single-copy reactions—that is, six reactions per Study member. We excluded Study members who only gave buccal swabs and/or are of Maori ancestry, leaving us with data from 829 Study members.
Additional Variables
Perinatal complications
We created a composite index of perinatal complications for each Study member by combining prenatal information drawn from hospital records with findings from a physical examination performed shortly after birth. The obstetric complications assessed in this Study have been described previously (Shalev et al., 2014), and include maternal diabetes, glycosuria, epilepsy, hypertension, eclampsia, antepartum hemorrhage, accidental hemorrhage, placenta previa, having had a previous small baby, gestational age younger than 37 weeks, birth weight less than 2.5kg, small for gestational age, major or minor neurologic signs, Rh incompatibility, ABO incompatibility, non-hemolytic hyperbilirubinemia, hypoxia at birth (idiopathic respiratory distress syndrome or apnea), and low Apgar score at birth. Based on evidence that the effects of adverse conditions are cumulative (Molfese, 2013), each condition was weighted equally and summed to yield an obstetric complications index. 650 Study members (63%) had 0 perinatal complications, 271 (26%) had 1 perinatal complication, and 116 (11%) had 2 or more.
Childhood ill health
Information about Study members’ childhood medical status was gathered every 2 years via standardized medical assessments and parent reports. Examinations included assessment by a neurologist, motor tests, and otological and opthalmological assessments. Parents were interviewed about milestones, accidents and poisonings, loss of consciousness, infections, and disease. In addition, home visits were conducted by a Health Department nurse, and a pediatrician conducted a general medical examination at the research unit. We compiled a “medical portfolio” for each child from birth to age 5 years, which was independently evaluated by two staff members who were blind to all other information about Study members. Each child’s health was coded on a 5-point scale (1 = “poor”, 5 = “excellent”), with inter-rater agreement = 0.85. Using this method, 686 children (66%) were rated as having health that was either “very good” or “excellent”.
Childhood SES
When Study members were born, we recorded parental SES on a scale that places occupations into one of six categories (1 = unskilled laborer, 6 = professional) based on education and income associated with that occupation in data from the New Zealand census. If both parents were employed, we used the higher occupation (M = 3.46, SD = 1.36).
Results
Consistent with literature identifying low intelligence as a risk factor for premature mortality (Calvin et al., 2011), the 30 Study members in our cohort who were deceased by age 38 scored about one half of a standard deviation below surviving cohort members on our measure of early-childhood intelligence, although this difference was not significant (d = 0.42, p = .15). Cohort members with present data for each of the four aging outcomes were representative of the 1,007 living cohort members with respect to early childhood intelligence (all p’s ≥ 0.22).
Does Intelligence Predict Study Members’ Rate of Aging?
At midlife, Study members with lower intelligence were biologically “older” than their same-age peers with higher intelligence (Table 2). Study members with higher intelligence had younger-looking faces, scored younger on the NHANES biomarker algorithm measure, had “younger” cardiovascular systems, and, to a lesser extent, longer telomeres. Results were similar regardless of whether intelligence was assessed concurrently with the biological age measure (when Study members were 38 years old), in middle childhood (when Study members were 7–11 years old), or in early childhood (when Study members were 3–5 years old). Effect sizes were comparable for perceived facial age, our biomarker algorithm, and heart age (r = 0.142–0.182), but were more modest for telomere length (r = 0.030–0.073).
Table 2.
Correlations Between Intelligence Assessed throughout the First Half of the Life Course and Biological Age at Age 38
| Intelligence measures and age of assessment | Measures of Aging | |||
|---|---|---|---|---|
| Perceived facial age | NHANES biomarker algorithm | Framingham heart age | Telomere length | |
| Early childhood (ages 3–5) | −0.160*** | −0.164*** | −0.182*** | 0.030 |
| Middle childhood (ages 7–11) | −0.161*** | −0.149*** | −0.142*** | 0.073* |
| Midlife (age 38) | −0.163*** | −0.173*** | −0.175*** | 0.059 |
| Verbal comprehension | −0.172*** | −0.140*** | −0.166*** | 0.042 |
| Perceptual reasoning | −0.101** | −0.158*** | −0.104** | 0.026 |
| Working memory | −0.117*** | −0.110** | −0.090** | 0.075* |
| Processing speed | −0.098** | −0.124*** | −0.189*** | 0.054 |
Note: Weschler Adult Intelligence Scale, 4th Edition (WAIS-IV) indices listed in italics. There were no significant sex differences in the associations between intelligence and biological aging. NHANES = National Health and Nutrition Examination Survey (III).
*p < .05. **p < .01. ***p < .001.
Because smoking is one of the constituent items used to calculate Framingham heart age and may influence our other outcome variables, we repeated these analyses using pack-years smoked as a covariate (a pack-year represents the number of cigarettes consumed during a year spent smoking 20 cigarettes per day). This adjustment left the pattern of results largely unchanged (Supplementary Table A).
Can the Association Between Early-Life Intelligence and Biological Age be Explained by Differences in Study Members’ Early Environments?
It is possible that the association between intelligence and biological age is driven partly by early educational experiences. Our data allowed us to investigate this possibility in two ways. First, we were able to examine Study members’ intelligence in early childhood, before they began formal schooling. Study members with lower intelligence at these early assessments were biologically older at midlife (Table 2). (Some Dunedin cohort members were enrolled in preschool by age 5, but this did not increase their tested intelligence; Silva, 1981.)
Second, the correlations between biological age and the components of intelligence that are more affected by schooling (e.g., verbal skills) were roughly equivalent to the correlations between biological age and the components of intelligence that are less affected by schooling (e.g., processing speed) (Table 2). This pattern also suggests that the intelligence-aging association is not simply a spurious artifact of education.
Is the Link Between Early-Life Intelligence and Aging Partly Attributable to Initial Differences in Early-Life Health or Early-Life SES?
Children with more perinatal complications performed significantly worse on early childhood intelligence tests (r = −0.131) and displayed more signs of aging (Table 3). Children with ill health in childhood showed a similar pattern, scoring lower on intelligence tests (r = 0.221) and “older” on measures of biological age (Table 3). Conversely, children born into upper-class families tended to perform better on intelligence tests than children from lower class families (r = 0.334), and scored “younger” on our measures of biological age (Table 3).
Table 3.
Correlations between Early Childhood Intelligence and Aging Measures Assessed at Age 38, Controlling for Potential Childhood Confounds
| Childhood confounds and age of assessment | Measures of aging | |||
|---|---|---|---|---|
| Perceived facial age | NHANES biomarker algorithm | Framingham heart age | Telomere length | |
| Perinatal complications (birth) | 0.110*** | 0.104** | 0.052 | −0.092** |
| Childhood Ill health (ages 3, 5) | −0.124*** | −0.072* | −0.108** | 0.012 |
| Childhood SES (birth) | −0.155*** | −0.091** | −0.102** | −0.014 |
| Early childhood intelligence (ages 3, 5) | −0.160*** | −0.164*** | −0.182*** | 0.030 |
| in subsamples | ||||
| With no history of perinatal complications (birth) | −0.171*** | −0.177**** | −0.225*** | 0.002 |
| With “very good” or “excellent” childhood health (ages 3, 5) | −0.106** | −0.164*** | −0.170*** | 0.030 |
| Born to middle-class families (birth) | −0.146** | −0.177*** | −0.245*** | 0.021 |
| controlling for | ||||
| Perinatal complications (birth) | −0.147*** | −0.152*** | −0.177*** | 0.020 |
| Childhood ill health (ages 3, 5) | −0.140*** | −0.154*** | −0.167*** | 0.028 |
| Childhood SES (birth) | −0.111** | −0.159*** | −0.164*** | 0.025 |
| All three potential confoundsa | −0.097** | −0.158*** | −0.163*** | 0.016 |
Note: Top panel: Correlations between potential childhood confounds and aging measures assessed at age 38. Middle panel: Correlations between early childhood intelligence and aging measures assessed at age 38 calculated in three restricted subsamples of Study members. Bottom panel: Correlations between early childhood intelligence and aging measures assessed at age 38 calculated in the full cohort, adjusted for perinatal complications, childhood ill health, and childhood SES. aStandardized betas from a general linear model using early childhood intelligence to predict each aging measure controlling for all three potential confounds. NHANES = National Health and Nutrition Examination Survey (III); SES = socioeconomic status.
*p < .05. **p < .01. ***p < .001.
To determine whether the associations between intelligence and aging outcomes are artifacts due to differences in our set of childhood confounds that pre-dated intellectual assessment, we first estimated associations between intelligence in early childhood and each of the midlife aging outcomes in two “utopian” subsamples (cf. Murray, 1998), one excluding all Study members with any history of perinatal complications whatsoever, and another including only Study members with “very good” or “excellent” childhood health. Despite reducing the sample size by almost half, Study members with lower intelligence in these healthy groups still tended to show signs of more advanced biological age (Table 3). In addition, we repeated our analyses in a subset of Study members who grew up in middle-class families (whose breadwinners had occupations such as building inspector, aircraft mechanic), excluding low-SES families (whose breadwinners had low-skill occupations such as foodpacker), as well as high-SES families (professional occupations such as dentist), thus precluding confounding by SES inequalities. The association between childhood IQ and our aging indicators again remained unaltered (Table 3).
As a further test, we again calculated correlations between Study members’ intelligence in early childhood and their scores on each midlife aging measure in the full cohort, but this time controlling for Study members’ histories of perinatal complications, childhood ill health, and childhood SES. Associations between early childhood intelligence and midlife biological age were largely unchanged (Table 3). Taken together, these findings support our hypothesis that the association between early life intelligence and aging cannot be directly attributed to differences in childhood health or SES that preceded intelligence test administration.
Discussion
In this longitudinal study of a birth cohort, we found that lower intelligence manifest as early as the preschool years (ages 3–5) was predictive of more advanced biological age measured more than three decades later. When followed up at age 38, Study members with lower intelligence looked older, scored as biologically older on a 10-biomarker algorithm reflecting metabolic, hepatic, renal, cardiovascular, pulmonary, and immune functioning, and had older cardiovascular systems—but not necessarily shorter telomeres. Moreover, our results suggest that the associations between intelligence and midlife biological age did not arise from early-life health problems or early socioeconomic disadvantage, and can be seen even when intelligence is assessed before the start of Study members’ formal education.
While previous studies have established a link between low intelligence and increased morbidity and mortality (Calvin et al., 2011; Der et al., 2009; Whalley & Deary, 2001), our study provides an initial demonstration that lower early-life intelligence may actually accelerate the aging process—and that evidence of this acceleration can be observed even in people assessed before the onset of most age-related disease. This finding suggests that accelerated aging may be one of the mechanisms linking low early-life intelligence to an array of negative, age-related health outcomes.
Our study has several methodological strengths. First, we tested intelligence repeatedly at different developmental stages and with different instruments, beginning as early as age 3. We found that the magnitude of the association between intelligence and biological age remained consistent across all assessment ages, possibly reflecting the long-term stability of intelligence throughout the life course. Second, our study included four distinct measures of accelerated aging: perceived facial age, biomarker-assessed biological age, heart age, and telomere length. Although smoking history is one of the variables used to calculate Framingham heart age, we were able to show that the associations between intelligence and our aging indicators were independent of this well-established risk factor. And third, the extraordinary retention rate of the Dunedin Study (with 95% of surviving Study members participating in the most recent assessment wave at age 38) allows us to largely avoid problems that commonly limit the generalizability of findings from longitudinal studies, such as selective attrition on the basis of intelligence (Salthouse, 2014).
Nevertheless, we acknowledge limitations. First, although we were able to rule out plausible artifactual explanations for why intelligence is associated with biological age (i.e., differences in early education, childhood health, and childhood SES), our data did not allow us to determine whether this association is causal. Second, our results were drawn from a single, largely Caucasian cohort born in the 1970s, and thus may not generalize to other populations. However, our results are consistent with findings connecting intelligence to health and mortality in other cohorts born in different time periods and in different countries (Arden et al., 2009; Wrulich et al., 2013).
Third, because we could examine only cross-sectional differences in biological age at midlife rather than change from an early-life baseline, it is possible that our midlife aging measures reflect stable individual differences rather than individual differences in change. In other words, less intelligent people may score higher on aging measures because they were biologically “older” from early life, rather than because they aged more rapidly. This hypothesis will need to be explored by studies with repeated measurements of aging indicators taken across the life course. Nevertheless, our observation that early-life intelligence predicts biological age independent of baseline differences in childhood health argues against the notion that intellectual differences predict biological age simply because less intelligent children are at greater risk of exhibiting poor health from birth.
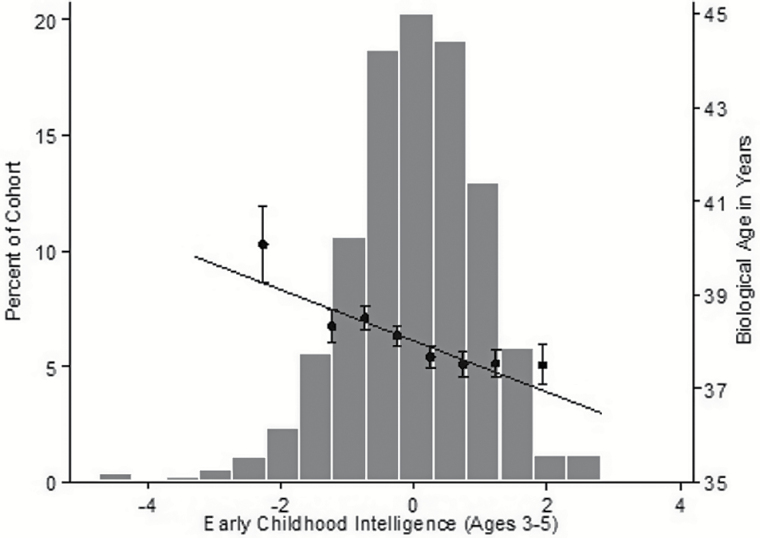
A fourth limitation, illustrated in Figure 1, is that the association between early-life intelligence and biological age had a relatively small effect size in the population as a whole (r = 0.1–0.2). For example, Study members who scored more than 1 SD above or below the cohort mean for early childhood intelligence differed in NHANES biological age by about 1 year (Figure 1). Although a year’s difference in biological age may not seem consequential for individuals in their late 30s, this difference may have greater practical significance in late life, as risk of mortality increases exponentially with age. Furthermore, because biological aging measures have stronger associations with mortality than chronological age, individual differences in biological age should exert more dramatic effects on age-related disease and mortality than equivalent differences in chronological age, particularly when such outcomes are considered at the population level.
Figure 1.
The association between early childhood intelligence and biological age as measured by the NHANES biomarker algorithm.
The histogram depicts the normal distribution of Study members’ early childhood intelligence scores, whereas the scatter plot and regression line show the association between early childhood intelligence and age 38 biological age as measured by the NHANES biomarker algorithm. The dots and standard error bars show average biological age for Study members with early childhood intelligence scores falling <−1.5, −1.5 to −1, −1 to −0.5, −0.5 to 0, 0–0.5, 0.5–1, 1–1.5, and > 1.5 SDs relative to the mean. NHANES = National Health and Nutrition Examination Survey (III).
Consistent with previous studies examining associations between telomere length and childhood intelligence (Harris, Martin-Ruiz, von Zglinicki, Starr, & Deary, 2012; Pearce et al., 2012), telomere length showed the weakest association with intelligence in our cohort. Interestingly, telomere length also showed only weak associations with our three other aging measures, which adds to existing evidence suggesting that the relationship between telomere length and “normal” aging parameters such as physical, sensory, and cognitive functioning is controversial (Sanders & Newman, 2013). The relatively weak associations between telomere length and intelligence seen here may also be due to differences in aggregation among our four outcome measures: Unlike perceived facial age, NHANES biomarker age, and Framingham heart age (which all combine either multiple variables or multiple ratings from independent observers), telomere length reflects a single indicator.
The reason(s) why early-life intelligence predicts biological age at midlife remain unclear. The literature connecting intelligence to health outcomes suggests at least four nonexclusive possibilities: First, the association between intelligence and biological age may arise because more intelligent people typically gain access to better health care, which may retard the aging process. Second, more intelligent people may obtain access to safer occupational and residential environments, which may, in turn, decrease their exposure to potentially age-accelerating conditions such as chronic job stress, dangerous working conditions, environmental toxins, and/or interpersonal violence. Third, intelligence may contribute to slower aging through several health-related behaviors such as sleep, physical activity, and dietary choices (Deary, Weiss, & Batty, 2010). And finally, intelligence may function as a measure of “brain health,” which reflects overall somatic integrity (Deary, 2012). Proponents of this last view have suggested that highly intelligent people age more slowly because of genetic factors such as a decreased mutation load (Arden et al., 2009) or pleiotropy at genetic loci associated with both higher intelligence and a longer lifespan (Dubal et al., 2014).
Aging is increasingly conceptualized as a unitary phenomenon that increases one’s risk of multiple age-related diseases simultaneously. Although life expectancy is increasing, people are living more years with disability from age-related conditions in 2010 than they were two decades ago (Murray et al., 2012). Identifying behavioral and psychological risk factors for accelerated aging thus constitutes a significant public health interest. Along with research demonstrating that early-life educational interventions can affect later health (Campbell et al., 2014), our study suggests the hypothesis that early-life cognitive enhancement interventions may help to decrease or delay age-related morbidity.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This work was supported by the National Institute on Aging (AG032282, AG048895), the Medical Research Council (MR/K00381X), the Economic and Social Research Council (ES/M010309/1), and the Jacobs Foundation. J. D. S. and D. W. B. were supported by the NIA (T32-AG000139-25, T-32AG000029), and (P30-AG028716-08).
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, their families, Study staff, and Study founder Phil Silva. We also thank Dana Kotter-Grühn, for her helpful comments on an earlier draft.
References
- Arden R. Gottfredson L. S., & Miller G (2009). Does a fitness factor contribute to the association between intelligence and health outcomes? Evidence from medical abnormality counts among 3654 US Veterans. Intelligence, 37, 581–591. doi:10.1016/j.intell.2009.03.008 [Google Scholar]
- Barker D. J. Osmond C. Golding J. Kuh D., & Wadsworth M. E (1989). Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ, 298, 564–567. doi:10.1136/bmj.298.6673.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty G. D. Deary I. J. Schoon I., & Gale C. R (2007). Mental ability across childhood in relation to risk factors for premature mortality in adult life: The 1970 British Cohort Study. Journal of Epidemiology and Community Health (1979-), 61, 997–1003. doi:10.1136/jech.2006.054494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty G. D. Gale C. R. Mortensen L. H. Langenberg C. Shipley M. J., & Deary I. J (2008). Pre-morbid intelligence, the metabolic syndrome and mortality: The Vietnam Experience Study. Diabetologia, 51, 436–443. doi:10.1007/s00125-007-0908-5 [DOI] [PubMed] [Google Scholar]
- Batty G. D. Shipley M. J. Gale C. R. Mortensen L. H., & Deary I. J (2008). Does IQ predict total and cardiovascular disease mortality as strongly as other risk factors? Comparison of effect estimates using the Vietnam Experience Study. Heart, 94, 1541–1544. doi:10.1136/hrt.2008.149567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D. W. Caspi A. Goldman-Mellor S. Meier M. H. Ramrakha S. Poulton R., & Moffitt T. E (2013). Is obesity associated with a decline in intelligence quotient during the first half of the life course? American Journal of Epidemiology, 178, 1461–1468. doi:10.1093/aje/kwt135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y., & Kuh D (2002). A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology, 31, 285–293. doi:10.1093/ije/31.2.285 [PubMed] [Google Scholar]
- Brinch C. N., & Galloway T. A (2012). Schooling in adolescence raises IQ scores. Proceedings of the National Academy of Sciences of the United States, 109, 425–430. doi:10.1073/pnas.1106077109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. N. Sprott R. Huber W. Bland J. Feuers R. Forster M., … Wolf N (2004). Biomarkers of aging: From primitive organisms to humans. The Journals of Gerontology, 59A, B560–7. doi:10.1093/gerona/59.6.b560 [DOI] [PubMed] [Google Scholar]
- Calvin C. M. Batty G. D. Lowe G. D., & Deary I. J (2011). Childhood intelligence and midlife inflammatory and hemostatic biomarkers: The National Child Development Study (1958) cohort. Health Psychology, 30, 710–718. doi:10.1037/a0023940 [DOI] [PubMed] [Google Scholar]
- Calvin C. M. Deary I. J. Fenton C. Roberts B. A. Der G. Leckenby N., & Batty G. D (2011). Intelligence in youth and all-cause-mortality: Systematic review with meta-analysis. International Journal of Epidemiology, 40, 626–644. doi:10.1093/ije/dyq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. Conti G. Heckman J. J. Moon S. H. Pinto R. Pungello E., & Pan Y (2014). Early childhood investments substantially boost adult health. Science, 343, 1478–1485. doi:10.1126/science.1248429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K. Thinggaard M. McGue M. Rexbye H. Hjelmborg J. v B. Aviv A., … Vaupel J. W (2009). Perceived age as clinically useful biomarker of ageing: cohort study. BMJ, 339, b5262. doi:10.1136/bmj.b5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino R. B. Vasan R. S. Pencina M. J. Wolf P. A. Cobain M. Massaro J. M., & Kannel W. B (2008). General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation, 117, 743–753. doi:10.1161/circulationaha.107.699579 [DOI] [PubMed] [Google Scholar]
- Deary I. J. (2010). Cognitive epidemiology: Its rise, its current issues, and its challenges. Personality and Individual Differences, 49, 337–343. doi:10.1016/j.paid.2009.11.012 [Google Scholar]
- Deary I. J. (2012). Looking for system integrity in cognitive epidemiology. Gerontology, 58, 545–553. doi:10.1159/000341157 [DOI] [PubMed] [Google Scholar]
- Deary I. J. Weiss A., & Batty G. D (2010). Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychological Science in the Public Interest, 11, 53–79. doi:10.1177/1529100610387081 [DOI] [PubMed] [Google Scholar]
- Deelen, J., Beekman, M., Codd, V., Trompet, S., Broer, L., Hägg, S., … Slagboom, P. E. (2014). Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. International Journal of Epidemiology, 43, 878–886. doi:10.1093/ije/dyt267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G. Batty G. D., & Deary I. J (2009). The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth. Intelligence, 37, 573–580. doi:10.1016/j.intell.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio B. M. Class Q. A. Rickert M. E. Larsson H. Långström N., & Lichtenstein P (2013). Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry, 70, 1231–1240. doi:10.1001/jamapsychiatry.2013.2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal D. B. Yokoyama J. S. Zhu L. Broestl L. Worden K. Wang D., … Mucke L (2014). Life extension factor Klotho enhances cognition. Cell Reports, 7, 1065–1076. doi:10.1016/j.celrep.2014.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. M. (1965). Expanded manual for the peabody picture vocabulary test. Minneapolis, MN: American Guidance Service. [Google Scholar]
- Harris S. E. Martin-Ruiz C. von Zglinicki T. Starr J. M., & Deary I. J (2012). Telomere length and aging biomarkers in 70-year-olds: The Lothian Birth Cohort 1936. Neurobiology of Aging, 33, 1486.e3–1486.e8. doi:10.1016/j.neurobiolaging.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Klemera P., & Doubal S (2006). A new approach to the concept and computation of biological age. Mechanisms of Ageing and Development, 127, 240–248. doi:10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Levine M. E. (2013). Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 68, 667–674. doi:10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C. Blasco M. A. Partridge L. Serrano M., & Kroemer G (2013). The Hallmarks of Aging. Cell, 153, 1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski D., & Humphreys L. G (1992). Some bodily and medical correlates of mathematical giftedness and commensurate levels of socioeconomic status. Intelligence, 16, 99–115. doi:10.1016/0160-2896(92)90027-O [Google Scholar]
- Mitnitski A., & Rockwood K (2013). Biological age revisited. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 69, 295–296. doi:10.1093/gerona/glt137 [DOI] [PubMed] [Google Scholar]
- Molfese, V. J. (2013). Perinatal risks across infancy and early childhood: What are the lingering effects on high and low risk samples? In L. F. DiLalla & S. M. C. Dollinger (Eds.), Assessment of biological mechanisms across the life span. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Murray C. A. (1998). Income inequality and IQ. Washington, DC: AEI Press. [Google Scholar]
- Murray C. J. L. Vos T. Lozano R. Naghavi M. Flaxman A. D. Michaud C., … Grant B (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380, 2197–223. doi:10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- Newcombe R. Milne B. J. Caspi A. Poulton R., & Moffitt T. E (2007). Birthweight predicts IQ: fact or artefact? Twin Research and Human Genetics, 10, 581–586. doi:10.1375/twin.10.4.581 [DOI] [PubMed] [Google Scholar]
- Pearce, M. S., Mann, K. D., Martin-Ruiz, C., Parker, L., White, M., von Zglinicki, T., & Adams, J. (2012). Childhood growth, IQ and education as predictors of white blood cell telomere length at age 49 -51 years: The Newcastle Thousand Families Study. PLoS One, 7, e40116. doi:10.1371/journal.pone.0040116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynell J. (1969). The Reynell developmental language scales. London, UK: National Foundation for Educational Research. [Google Scholar]
- Richards M. Strachan D. Hardy R. Kuh D., & Wadsworth M (2005). Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine, 67, 602–608. doi:10.1097/01.psy.0000170337.51848.68 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2014). Selectivity of attrition in longitudinal studies of cognitive functioning. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69, 567–574. doi:10.1093/geronb/gbt046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J. L., & Newman A. B (2013). Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiologic Reviews, 35, 112–131. doi:10.1093/epirev/mxs008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev, I., Moffitt, T. E., Braithwaite, A. W., Danese, A., Fleming, N. I., Goldman-Mellor, S., … Caspi, A. (2014). Internalizing disorders and leukocyte telomere erosion: A prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Molecular Psychiatry, 19, 1163–1170. doi:10.1038/mp.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, P. (1981). Developmental and educational experiences and activities. In P. Silva, R. McGee, & S. M. Williams (Eds.), From birth to seven: Child development in Dunedin: A multidisciplinary study. Otago, NZ: Otago University Press. [Google Scholar]
- Strand B. H. Groholt E.-K. Steingrimsdottir O. A. Blakely T. Graff-Iversen S., & Naess O (2010). Educational inequalities in mortality over four decades in Norway: Prospective study of middle aged men and women followed for cause specific mortality, 1960–2000. BMJ, 340, c654. doi:10.1136/bmj.c654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman L. M., Merrill M. A. (1960). Stanford-Binet intelligence scale: Manual for the third revision form L-M. Boston, MA: Houghton Mifflin. [Google Scholar]
- Von Stumm S., & Plomin R (2015). Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence, 48, 30–36. doi:10.1016/j.intell.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1974). Manual for the Wechsler Intelligence Scale for Children, Revised. New York, NY: Psychological Corp. [Google Scholar]
- Wechsler D. (2008). Wechsler Adult Intelligence Scale (4th ed). San Antonio, TX: Pearson Assessment. [Google Scholar]
- Whalley L. J., & Deary I. J (2001). Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ, 322, 819–822. doi:10.1136/bmj.322.7290.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrulich M. Brunner M. Stadler G. Schalke D. Keller U. Chmiel M., & Martin R (2013). Childhood intelligence and adult health: The mediating roles of education and socioeconomic status. Intelligence, 41, 490–500. doi:10.1016/j.intell.2013.06.015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.