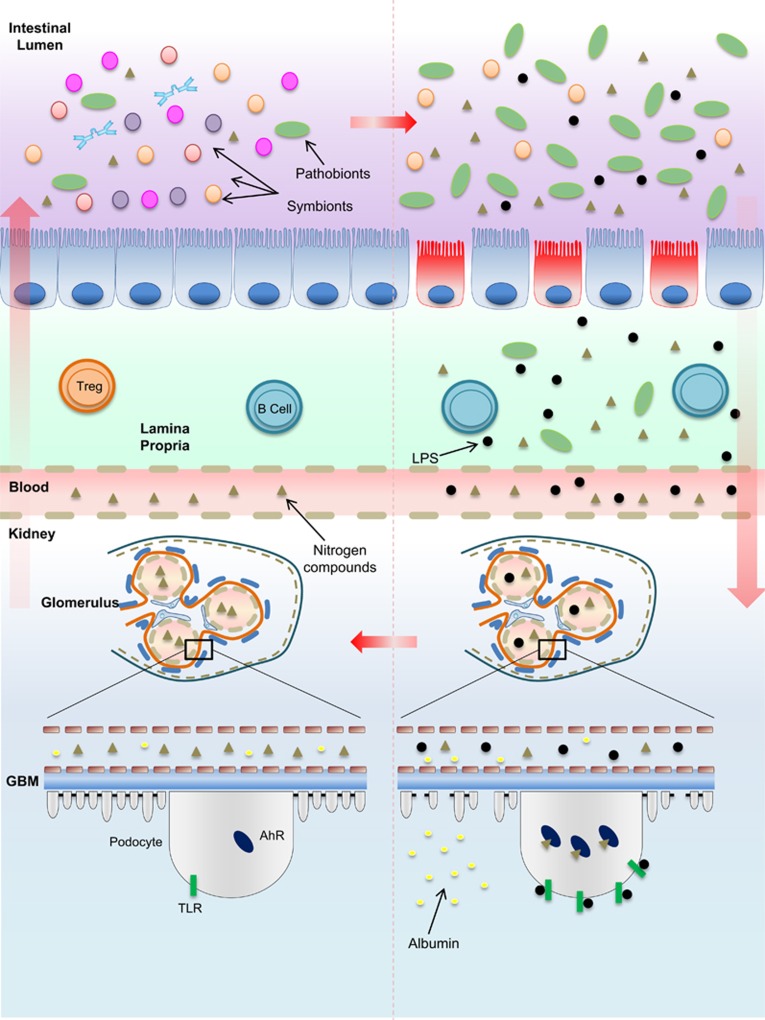
Figure 2.
Dybiosis and the evolution of kidney damage. In a healthy intestinal microflora, symbiotic bacteria coexist with pathogenic bacteria. The outnumbered presence of symbionts is supposed to maintain gut homeostasis by degrading resistant carbohydrates and proteins that escape from gastric metabolism. An imbalance of the gut biochemical milieu either due to the changes in dietary habits, in which the supply of non-metabolized proteins that reach the intestine is higher than the supply of carbohydrates, or due to the biochemical alterations resulting from reduced kidney function, which impairs the excretion of metabolism products such as urea or acid, may reinforce or contribute to dysbiosis. The reduction of mucus production, loss of tight junctions, leakage of the gut barrier and bacterial translocation initiated due to the overproduction of nitrogen products by urease and uricase-producing bacteria generate high levels of indole sulfate and p-cresil, which, along with endotoxins and LPS, cross the lamina propria to reach the kidneys. The activation of TLRs and other pattern recognition receptors by endotoxins as well as the binding of AhRs by indoxyl sulfate in podocytes are involved in podocyte death and, consequently, the loss of renal selectivity, increased permeability to high-molecular-weight proteins, such as albumin, and renal failure. The damage is propagated to other cells on subjacent glomeruli and progressively contributes to decreased renal clearance. Higher levels of nitrogen products in the blood achieve the intestinal lumen and reinforce, in a vicious cycle, dysbiosis. AhR, aryl hydrocarbon receptor; GBM, glomerular basement membrane. LPS, lipopolysaccharide; TLR, Toll-like receptor; Treg, regulatory T cell.