Fig. 1.
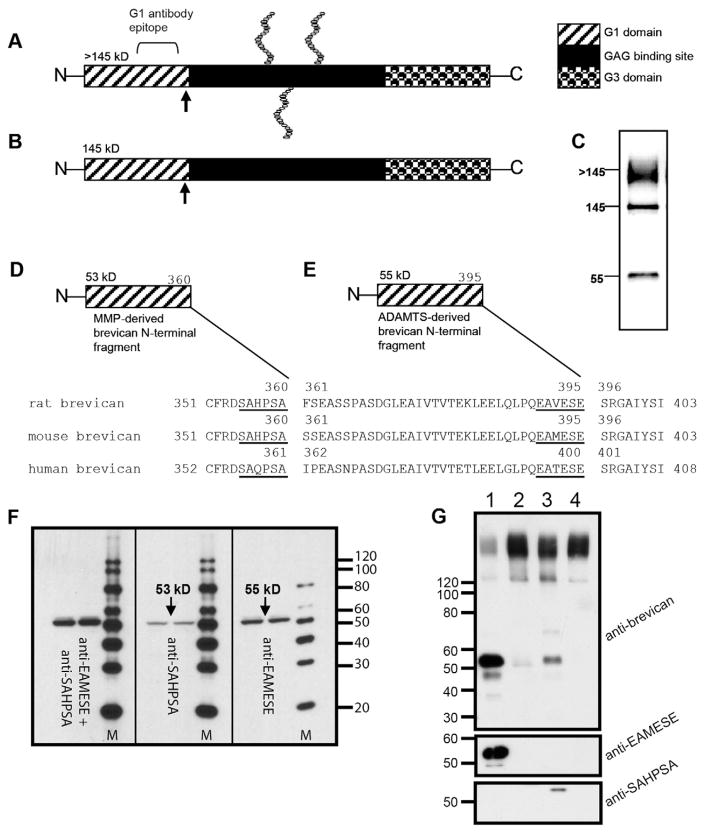
Detection of brevican isoforms and proteolytic degradation by endogenous proteases at specific cleavage sites. Brevican is secreted as a >145 kDa protein bearing 1–3 CS chains (A). Brevican is also secreted as the holoprotein without CS chains at 145 kDa (B). When probed on Western blot with an N-terminal antibody (BD Biosciences, San Jose, CA) three immunoreactive bands appear: a >145 kDa smear (glycosylated brevican), the 145 kDa core protein, and a ~55 kDa proteolytic fragment. Arrows in (A) and (B) indicate proteolytic cleavage sites. Fragments of brevican are generated by endogenous proteases, the MMPs (D) and ADAMTSs (E). Each has a distinct, specific cleavage site sequence on the brevican protein. Shown here are the specific cleavage sequences for the MMPs and ADAMTSs in mouse, rat and human brevican (based on data from [51]). The MMP cleavage-site is 35 amino acid residues upstream from the ADAMTS-specific site (D and E). Distinct “neoepitope” antibodies recognize the MMP- and ADAMTS-derived cleavage fragments of brevican in mouse brain extracts subjected to Western blot on 4–20% gradient SDS-PAGE gels (F). Anti-SAHPSA recognizes the 53 kDa, MMP-derived fragment of brevican (F; middle panel) whereas anti-EAMESE detects the 55 kDa, ADAMTS-derived form (F; right panel). Mixing the two antibodies detects a “thicker” band in this region (F; left panel). “M” indicates molecular weight markers in (F). Antibodies recognize distinct products after proteolytic cleavage with hrADAMTS4 or hrMMP-2 (G). Proteoglycan purified from the mouse brain was incubated with 50 nM hrADAMTS-4 (G, lane 1), 50 nM hrADAMTS4 + 5 mM EDTA (G, lane 2), 50 nM hrMMP-2 (G, lane 3) or 50 nM hrMMP-2 + 5 mM EDTA (G, lane 4) and immunoblotted for brevican. Note that the ADAMTS-derived brevican fragment was selectively recognized by anti-EAMESE and the brevican MMP product was recognized by anti-SAHPSA (Figure used with permission from Journal of Neurochemistry [52]).