Abstract
Research on human immune responses frequently involves the use of peripheral blood mononuclear cells (PBMC) immediately, or at significantly delayed timepoints, after collection. This requires PBMC isolation from whole blood and cryopreservation for some applications. It is important to standardize protocols for blood collection, PBMC isolation, cryopreservation, and thawing that maximize survival and functionality of PBMC at the time of analysis. This resource includes detailed protocols describing blood collection tubes, isolation of PBMC using a density gradient, cryopreservation of PBMC, and thawing of cells as well as preparation for functional assays. For each protocol, we include important considerations, such as timing, storage temperatures, and freezing rate. In addition, we provide alternatives so that researchers can make informed decisions in determining the optimal protocol for their application.
Studies of the human immune system often involve isolation of peripheral blood mononuclear cells (PBMCs), and appropriate protocols for isolation and storage of PBMCs are important to facilitate this research. Here, we provide standard operating protocols for the acquisition, isolation, freezing, storage, and thawing of PBMC.
Obtaining live blood cells for analysis requires the use of anticoagulants during blood collection. The choice of collection tube depends on the ultimate downstream applications. Commonly used anticoagulants include ethylenediaminetetraacetic acid (EDTA) (in purple or lavender top vacutainers), acid citrate dextrose (ACD) (yellow top vacutainers), sodium citrate (light blue top vacutainers), and sodium heparin (green top vacutainers). Because additives used in blood collection can affect the suitability of the cells for downstream assays, careful consideration should be made in deciding which type of vacutainers to use for blood collection. For example, EDTA has been shown to interfere with antigen-specific T-cell responses by some,1 but not others2 when compared with blood collected using ACD or heparin. Sodium heparin binds to DNA and interferes with many enzymes used for molecular analyses of DNA therefore should be avoided when the cells are destined for gene expression analyses.3 Cyto-Chex tubes contain fixatives that preserve the blood cells for later flow cytometric analysis; however, fixatives in Cyto-Chex tubes kill cells and can also destroy certain antigens for flow cytometric detection.4
The most commonly used method to isolate PBMCs from blood is density gradient centrifugation using Ficoll. To achieve high yield and purity of cells with reproducibility, multiple parameters need to be considered, such as blood storage condition before processing, choice of blood diluent, Ficoll underlay/overlay technique, and centrifugation conditions. Special blood collection tubes are also available to reduce the variability, such as cell preparation tubes (CPT), prepackaged with a polyester gel and density gradient medium so that they can be directly centrifuged to separate PBMCs from RBC and granulocytes and plasma. The CPT tubes are available with the various anticoagulants as described above.
There are several reasons to store samples for in vitro studies including the need to batch analyses,5 and to preserve samples for analyses that may not be immediately possible. When storing PBMC, time, and temperature before PBMC isolation, between isolation and freezing, rate of freezing, duration of cryopreservation, and storage temperature can all affect cell viability and functionality. Before deciding to cryopreserve cells, it is important to consider what is most important to the experiment at hand. For example, some cellular functions6,7 and phenotypes8 can be affected by cryopreservation, so for such studies, the function may be more important than being able to batch cells.
When thawing PBMC, it is also important to know appropriate media and timing to minimize cell damage and death.9 It is critical to warm quickly and briefly to minimize osmotic damage and toxic effects of dimethylsulfoxide (DMSO), and then to transfer the cells rapidly into appropriate media. In addition, timing of thawing matters for downstream applications. If the thawed PBMCs are to be used for a functional assay, a rest at high cell concentrations for several hours to overnight is often helpful.10
There is a great deal of variation in the protocols for each step, often without data to support 1 approach versus another. The goal of this resource is to help researchers identify the most important factors for their experiments and provide them with the available data on selecting PBMC preparation and storage protocols so that they can standardize their assays and have confidence in their results.
Collection of Human Blood
Method
-
(1)
Collect blood specimen by venipuncture and follow recommendations according to guidelines established by the Clinical and Laboratory Standards Institute11 Because blood collection tubes contain chemical additives, it is important to avoid possible backflow from the tubes. To guard against backflow:
-
(a)
Keep patient's arm in the downward position during the collection procedure.
-
(b)
Hold the tube with the stopper in the uppermost position so that the tube contents do not touch the stopper or the end of the needle during sample collection.
-
(c)
Release tourniquet once blood starts to flow in the tube, or within 2 minutes of application.
-
(a)
-
(2)
Fill tube completely.
-
(3)
Remove tube from adapter and immediately mix by gentle inversion.
Critical Parameter
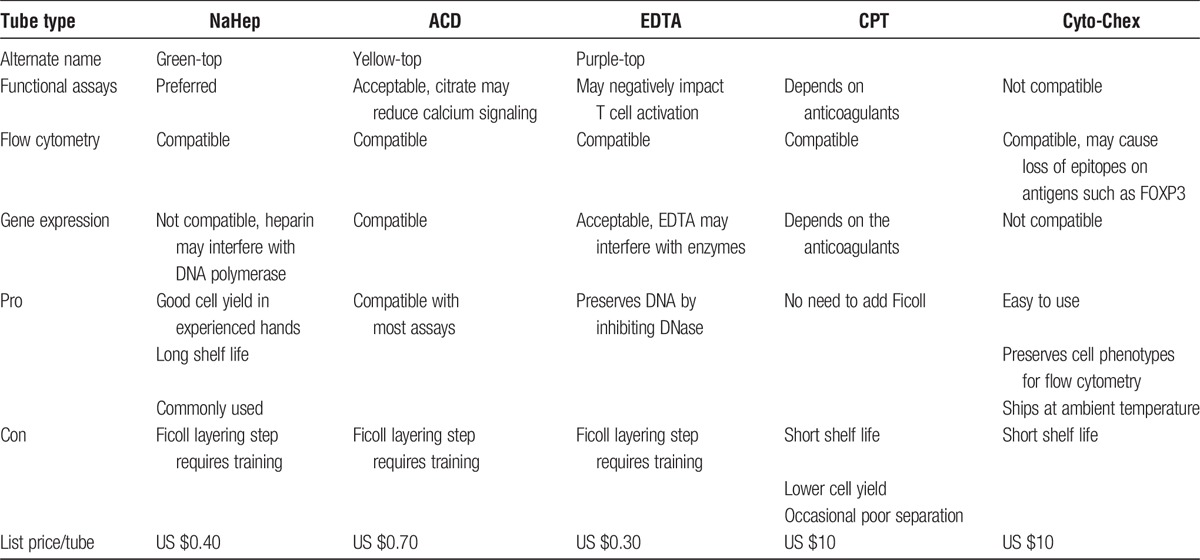
It is important to select the right type of blood collection tubes based on the purpose of planned downstream applications. Table 1 shows a comprehensive comparison among currently available types of blood collection tubes including assay compatibility and pros and cons.
TABLE 1.
Summary of types of blood collection tubes and their pros and cons
Isolation of Human PBMC Using Density Gradient Centrifugation
Materials
-
(1)
Sterile Ca++- and Mg++-free saline solution such as Dulbecco phosphate-buffered saline or Hanks balanced salt solution.
-
(2)
Ficoll-Paque (density 1.077 ± 0.001 g/mL at 20°C) (GE Healthcare).
Methods
-
(1)
Obtain 10-mL tubes of patient blood in the appropriate type of collection tubes (see Table 1 for guidance). If the blood cannot be processed immediately, store it at room temperature (RT) on a rocker and process within 24 hours; however, it is always best to process immediately (see Critical Parameters section).
-
(2)
Gently invert the tubes 8 times to mix the blood and use a 10-mL serological pipette to transfer 10 mL of blood to a 50-mL conical tube. Rinse each blood tube with 10-mL sterile saline solution and add to the 50-mL conical tube. There should be approximately 20 mL of blood/phosphate-buffered saline in each tube.
-
(3)
Using a 10-mL serological pipette carefully underlay the diluted blood with 10 mL of Ficoll (at RT) being careful to avoid mixing the 2 layers. Do not dispense Ficoll from the pipette until the tip is at the bottom, and angle it slightly to avoid pressure on the base of the tube. Set the pipette speed to slow for the first 5 to 6 mL to avoid mixing layers. Stop dispensing Ficoll when there are about 500 μL left in the tip of the pipette to avoid blowing bubbles. Slowly withdraw the pipette from the tube, and the final 500 μL of Ficoll will release into the tube through gravity.
-
(4)
Centrifuge at 800g for 30 minutes at RT with the brake turned OFF (total time ~40 min). Do not let the cells sit in the centrifuge after spinning. Note: If cells are left overnight before isolation, it is preferable to centrifuge at same speed for 40 minutes.
-
(5)
Using a sterile transfer pipette, harvest the buffy coat into a fresh 50-mL conical tube. The buffy coat is the mononuclear cell band sitting between the diluted plasma (yellow with phosphate-buffered saline dilution, pink with Hanks balanced salt solution dilution) top layer and the Ficoll (clear/red) bottom layer (Figure 1). When the border between these 2 layers is sharp, all cells have been harvested. Avoid collecting excess Ficoll (below) along with the cells but it is okay to harvest plasma (top layer).
-
(6)
Estimate the volume of the transferred mononuclear cells. Add at least 3 volumes of sterile saline solution to the mononuclear cells in the centrifuge tube. Gently invert tube to mix.
-
(7)
Centrifuge at 310 to 400g for 10 to 15 minutes at RT with the brake ON (as per manufacturer's recommendation).
-
(8)
Remove supernatant and resuspend the pellet by gently tapping the side of the tube. Add 6 to 8 mL of sterile saline solution to each tube and resuspend cells by gentle drawing in and out of pipette.
-
(9)
Centrifuge at 310 to 400g for 10 minutes at RT with the brake ON.
Note: If it is important to also get rid of platelets, reduce centrifugation speed for this step to 129g. The lower centrifugation speed prevents pelleting of platelets,12 but the PBMC pellet will also be loose. Therefore, it is important to remove the supernatant immediately after the spin, using a pipette. When removing media, leave approximately 1 mL behind. Decanting or waiting risks loss of cells.
-
(10)
Remove supernatant and resuspend in appropriate media for the application.
-
(11)
Count the cells using Trypan Blue and the hemocytometer or an automated cell counter (per manufacturer’s instructions). The accuracy of this count is extremely important because we will use it to calculate leukocyte subpopulations so please be consistent. If RBC contamination is a concern (see Troubleshooting), repeat the count with another cell aliquot using one of the following methods
-
(a)
Addition of acetic acid lyses RBC.
-
(b)
Stain with a mixture of acridine orange (AO) and ethidium bromide (EtBr) or propidium iodide (PI)13 and count the cells using a microscope equipped with a ultraviolet lamp.
Note: AO is a membrane-permeable dye that stains nucleated viable cells green, and EtBr and PI are membrane-impermeable and stain nonviable nucleated cells. AO and EtBr/PI do not stain RBCs because they are non-nucleated cells.
-
(a)
-
(12)
If freezing the cells, move on to the cryopreservation protocol below. If not, spin the 15-mL tubes 1 final time at 310 to 400g for 6 to 7 minutes with the brake ON and prepare for downstream applications.
Alternative: There are several commercially available approaches to isolation of PBMCs that can be used instead of Ficoll. These could be used according to their manufacturer's instructions.
FIGURE 1.
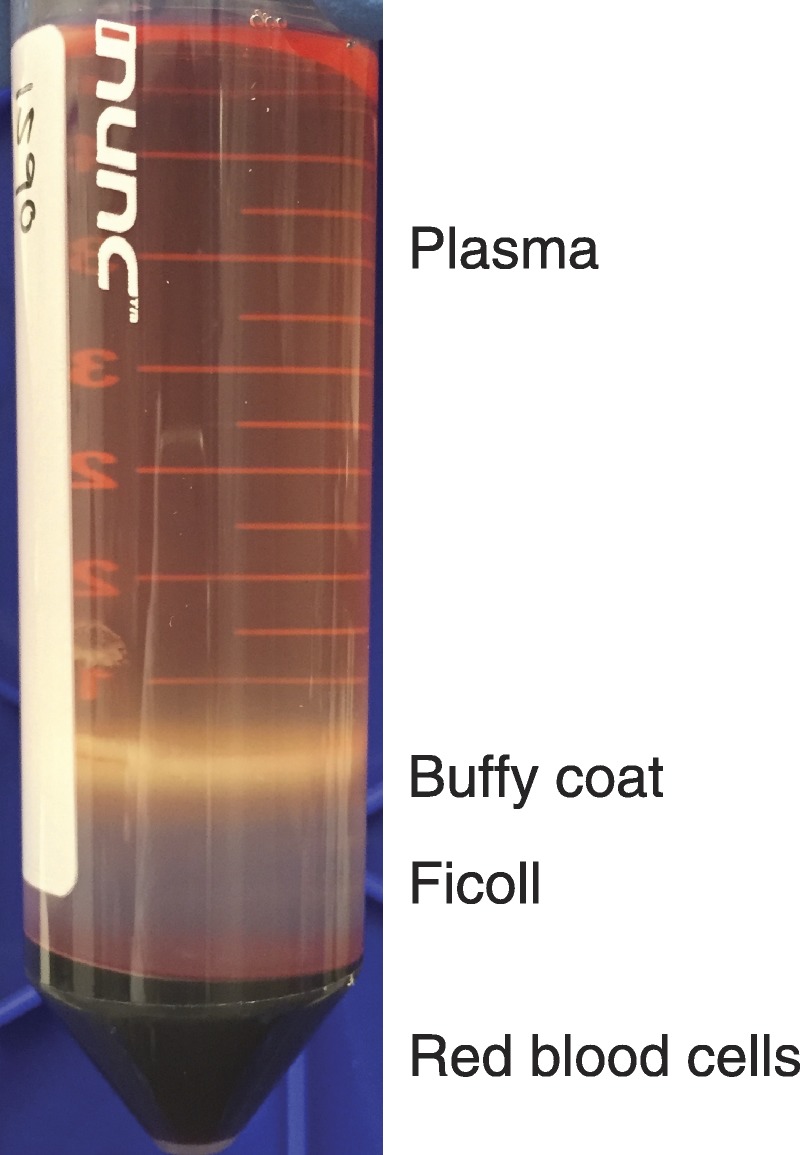
Photograph of Ficoll layering. Image depicts a 50-mL conical tube after centrifugation for Ficoll layering. PBMC are in the white layer between the plasma and Ficoll.
Cryopreservation of Human PBMC
Materials
-
(1)
Laminar flow biosafety cabinet
-
(2)
−80°C freezer
-
(3)
Liquid nitrogen (−140°C) storage unit
-
(4)
Cell freezing devices such as Mr. Frosty (Nalgene or equivalent), CoolCell (Biocision), or electronic controlled rate freezer (Thermo or equivalent).
-
(5)
Previously isolated/quantitated human PBMC
-
(6)
DMSO (eg, Sigma, cat D2650)
-
(7)
Heat-inactivated14 fetal bovine serum (FBS)
-
(8)
Cryovials (2 mL size) (eg, Corning Inc. cat 430659)
-
(9)
Labels
Methods
This protocol contains 2 alternative versions, both of which involve a final cryopreservation medium of FBS with 10% DMSO. The first method involves direct resuspension of cell pellets in FBS with 10% DMSO, with cryopreservation media at RT. The second involves resuspension of cell pellets in 50% of the required volume in 100% FBS, followed by addition of 2× cryopreservation media (FBS with 20% DMSO) at 4°C. Where the protocols diverge, the second protocol is explained in italics. The rationale for both is explained under Considerations and Alternatives.
-
(1)
Prepare cryopreservation media
-
(a)
Thaw FBS.
-
(b)
Mix FBS and DMSO in a 9:1 ratio, for example, 90 mL FBS + 10 mL DMSO and filter sterilize.
Alternative protocol: prepare 2× cryopreservation media (40 mL FBS + 10 mL DMSO; and 50 mL 100% FBS in separate tube).
-
(c)
Label the bottle and date. Cryopreservation media may be stored at 4°C for up to 1 month, or frozen in single use aliquots at −20°C.
-
(d)
For immediate use, cryopreservation media should be at RT. To minimize unnecessary warming of media, you can approximate the amount based on sample number and cell count. Calculate 1 mL per aliquot that contains up to 10 × 106 cells plus 1 to 2 mL extra.
Alternative protocol: use cryopreservation media at 4°C.
-
(a)
-
2.
Label Cryovials
-
(a)
To estimate number of vials, in general, a single cryovial should contain between 5 and 50 × 106 isolated PBMC in 1 mL of freezing media, depending on the downstream application.
-
(b)
Label vials with at minimum: study name/number, investigator, subject number, date specimen was collected from the subject, and cell quantity.
-
(a)
-
(3)
Resuspend PBMC in cryopreservation media
Cells should be resuspended in cryopreservation solution for as short a time as possible and no longer than 2 to 3 minutes before being placed into the freezer. This may require small batches of samples.
-
(a)
Centrifuge counted cells at 310g in a swinging bucket rotor at RT. Remove supernatant and gently break up pellet by tapping the side of the tube.
-
(b)
Using a 10-mL sterile pipet, suspend the PBMC in an appropriate amount of RT cryopreservation media.
Alternative protocol: Resuspend cells in 50% final volume of FBS (ie, if final volume is 5 mL, resuspend in 2.5 mL) and leave on ice for 10 to 15 minutes. Add equal volume of 2× cryopreservation solution (See critical parameters for a discussion of cryopreservation solution temperature). The cryopreservation solution is added drop by drop over a period of ~2 minutes, with the pipette tip against the wall of the conical tube. Mix cells by swirling as solution is added.
-
(c)
Immediately aliquot 1 mL of suspended cells (final concentration of 5 to 50 × 106/mL) into prelabeled cryovials. Cap vials tightly and place into a container for freezing, such as Mr. Frosty or CoolCell. Follow manufacturer's instructions for use of freezing container. The freezing container should be at RT when the cryovials are placed in it. Transfer to −80°C freezer.
-
(a)
-
(4)
Long-term storage
-
(a)
Keep cells at −80°C overnight but no longer than 7 days.
-
(b)
Determine final placement of samples before starting the transfer. It is important to not allow samples to warm up.
-
(c)
Place a generous amount of dry ice in an ice bucket. Handle cryovials by the tops only to allow frozen sample to stay cold and hold on dry ice while transferring to final liquid N2 destination.
-
(d)
Keep the final storage box as cold as possible—in vapor phase of liquid N2 if possible or on dry ice. Transfer cryovials and replace storage box into liquid N2.
-
(a)
Thawing of Cryopreserved Human PBMC
Standard Operating Procedure
Materials
-
(1)
Laminar flow biosafety cabinet
-
(2)
Water bath set at 37°C or an electronic thawing device such as Thawstar (Biocision)
-
(3)
Benchtop centrifuge with swinging bucket rotor at RT
-
(4)
Frozen PBMC in cryovial—stored in liquid nitrogen (−140°C) storage unit.
-
(5)
RPMI
-
(6)
R10 cell wash/growth media: RPMI + 10% FBS, 1% l-glutamine, 1% pen/strep and sterile filtered
-
(7)
DNase (eg, Roche DNase I recombinant, 04 716 728 001)
-
(8)
70% Ethanol in squirt bottle
Methods
-
(1)
Prepare media
-
(a)
Prepare RPMI/DNase for thawing and R10/DNase for resuspension immediately before thawing cells.
-
(i)
R10 without DNase can be kept at 4°C for up to 1 month.
-
(ii)
For each cryovial, 15 mL RPMI/DNase and 5 to 10 mL R10/DNase will be needed.
-
(iii)
For thawing, RPMI/DNase should be prepared at a concentration of 1 unit DNase (0.01 μL of 10 000 U/mL) per mL. Sterile filter.
-
(iv)
Aliquot 9 mL RPMI/DNase into a 15-mL conical for each cryovial and label.
-
(v)
Prepare R10/DNase at 10 units DNase/mL and sterile filter.
-
(vi)
Warm RPMI/DNase and R10/DNase to RT.
-
(i)
-
(a)
-
(2)
Thaw cells
-
(a)
Make sure that everything is ready in the hood—9 mL aliquots of RPMI/DNase, pipettes, and so on. Check that the water bath is at 37°C.
-
(b)
Keep all cryovials on dry ice until ready to thaw.
-
(c)
Hold a cryovial by the cap, partially submerged in a 37°C water bath. Move vial back and forth slowly. The objective is to thaw the vial to the point where there is some liquid, but the ice ball in the center is still present (usually 15-45 seconds). If it is not obvious when the vial reaches this point, a good test is whether the ice ball detaches from the base of the tube.
-
(d)
Do not shake or flick the partially thawed vial—cells are very fragile at this point.
-
(e)
Remove the vial from the water bath and quickly spray with 70% ethanol. Blot dry with paper towel.
Alternative to thawing in water bath, an electronic thawing device such as Thawstar can be used.
-
(a)
-
(3)
Wash cells
-
(a)
In the Biosafety cabinet, uncap the vial and use a 2-mL serological pipet to add drop by drop 1 mL of warmed (RT) RPMI/DNase to the cryovial. The ice ball should quickly melt and cells are then transferred directly to the 15 mL conical tube with 9 mL of RPMI/DNase aliquoted for that sample. Rinse the cryovial once, then add RPMI/DNase to 15 mL total.
-
(b)
Repeat for other vials to be thawed.
-
(c)
Remove 10 to 50 μL aliquots for counting and count during step d. Note: This method works well for 1 to 2 samples. When thawing multiple samples, do the whole process (thawing through resuspension) for 2 tubes at a time. It is also acceptable to spin down, then count, then spin again. However, each spin step can result in a loss of cells, so minimizing spins whenever possible is helpful. Waiting until after a spin to count may improve count accuracy, so in cases where counts are critical, counting after spinning is acceptable.
-
(d)
Centrifuge at 310g for 7 to 10 minutes at RT.
-
(e)
Pour off supernatant
-
(f)
Loosen cell pellet by tapping tube gently. Resuspend cells to 2 × 106 cells/mL by gentle pipetting.
-
(g)
If cells are being used in a functional assay, rest the cells for several hours to overnight in R10 medium at 2 to 3 × 106 cells/mL.
-
(i)
37°C/5% CO2 incubator. This can be done in T25 flasks or in 6-, 12-, or 24-well plates depending on the cell counts.
-
(ii)
Count cells again before the functional assay, as there may be some cell death after rest.
-
(i)
-
(a)
Considerations and Alternatives
Troubleshooting
-
(A)
Inaccurate counts due to RBC contamination during PBMC isolation: This is most likely to be a problem for samples left overnight before processing, because these samples tend to have less separation between PBMC and RBC in the Ficoll gradient. Red blood cells are round with thicker outlines than other cells. At high magnifications, it may be possible to see their biconcave shape. Lysis with ACK (ammonium-chloride-potassium) buffer can reduce the frequency of RBC in the sample and using the alternative counting methods outlined in the protocol can improve the accuracy of the counts.
-
(B)
Cell death after thaw: Typically, 10% to 15% of cells die with the freeze-thaw procedure. Cell death during thawing may result in leakage of viscous DNA, cell clumping, and low cell recovery. Inclusion of DNase in wash media will prevent clumping of the cells and improve cell recovery. Alternative: If DNase is problematic for downstream applications (eg, studies involving addition of DNA or DNA binding proteins), an acceptable alternative to addition of DNase to media is to filter cells through a 70-μm filter.
-
(C)
RBC present in thawed cells: If the pellet of thawed cells is visibly red, lyse RBC with ACK buffer. Do not do so more than once, as that is likely to result in a lot of cell death, particularly with recently thawed cells. Most RBCs are lysed and die during the freeze thaw process so if there are few RBCs before freezing, lysis is unlikely to be necessary.
-
(D)
Low yield of cells upon thawing: This is often due to poor handling before freezing, such as extended time with cells in cryopreservation media at RT or using old freezing media. It may also be due to leaving cells at −80°C for too long before moving to LN2. Handling during freezing, storage, and thawing are further discussed in critical parameters (below).
-
(E)
High cell death after rest: This often results if the cell numbers were unexpectedly low before the rest—low cell numbers are indicative of poor survival during freezing and thawing, and those that remain are likely to be in poor condition. This can also result from inappropriate protocol for resting the cells (eg, 2 mL in a flask).
Time considerations
-
A)
For freezing: The freezing protocol can take as little as 30 minutes, once the media is ready. Controlled rate freezing (see critical parameters) can take up to 2 hours. Because of the importance of moving the cells to the freezer immediately after resuspension in media containing DMSO, it is crucial to allot sufficient time to complete the procedure without stopping.
-
(B)
For thawing: The main time considerations for thawing are to have everything ready before removing the cryovials from LN2, and to rapidly transfer cells from the cryovial to the tube containing media.
-
(C)
For functional assays: the rest of thawed cells is recommended to allow the cells to recover from the stress of cryopreservation. If the functional assay calls for an overnight or longer stimulation, it works well to start thawing the morning, rest the cells for several hours during the day, and start the stimulation at the end of the day. If shorter 4- to 6-hour stimulation is called for, it may be better to thaw cells later in the day, rest overnight so that the stimulation can start in the morning the next day.
Critical Parameters
There are many published protocols on preparation, freezing, and thawing of PBMC, which have many similarities but also differ in a variety of ways. These differences include composition of freezing media, temperature of freezing media, and time of exposure to DMSO. The critical parameters below comprise a list of the factors that we have found to be necessary to successful freezing and thawing of viable cells.
-
(A)
Blood storage before processing: It is best to process the blood soon after collection. Longer storage is correlated with more granulocyte and/or RBC contamination and reduced response of T cells. When shipment and storage is a must, it is best to keep the cells at RT. Lower (4°C) temperatures cause higher contamination of granulocytes after Ficoll separation. There are reports that human Th17 T cells do not survive if whole blood is cooled to 4°C before PBMC isolation (Chong and Alegre, personal communication). Gentle agitation on a rocker helps to reduce granulocyte contamination.6
-
(B)
Recovery of cells when using a Ficoll gradient: Isolation of PBMC using Ficoll can produce variable results, especially when the protocol is completed by inexperienced personnel.6 In addition, there are reports that Ficoll gradient isolation can have selective loss of some immune cell types,15,16 so it is advisable when designing a assay to make sure that the isolation does not eliminate the cells of interest.
-
(C)
Optimization of cell counting: manual counting with a hemocytometer is relatively inexpensive, and also has the advantage of visual inspection of the cells. However, manual counting varies from person to person, so all counting should be conducted by one individual. Automated cell counters are another option, but accuracy should be compared with careful manual counting before relying on an automated counter. In addition, it is important to select a counter that allows discrimination between live and dead cells and use methods specific for the individual counter.
-
(D)
Quality of cryopreservation media: Regular preparation of fresh cryopreservation media is recommended due to the tendency of DMSO to oxidize to toxic substances. We recommend using DMSO in previously prepared single use aliquots or supplied in sealed single-use ampules instead of bottles for repeated use. Fetal bovine serum should also not be stored at 4°C for longer than 1 month, due to the potential for degradation or contamination. We observed that 10% DMSO in FBS provides improved viability and recovery when compared with cryomedium made of 10% DMSO in various concentrations of human AB serum (data not shown). When animal products are not permissible for downstream applications due to concerns of antigens present in FBS or need for good manufacturing practice compliance, commercial serum-free medium can be used, such as CS5 (5% DMSO) and CS10 (10% DMSO) from Biolife.
-
(E)
1× versus 2× cryopreservation media: The use of 2× cryopreservation media after resuspension in FBS allows for better control of the time the cells spend in DMSO, avoids direct resuspension in DMSO, and allows for cooling of the cells before addition of DMSO (see “Freezing rate” below). However, many researchers have successfully cryopreserved cells with direct resuspension in FBS with 10% DMSO. Exposure to 10% DMSO for up to an hour is not toxic, but it quickly becomes toxic after that,17 which is why cells need to be transferred to a freezer quickly after addition of DMSO. We have observed similar viability of cells frozen with 1× and 2× cryopreservation media (data not shown).
-
(F)
Cell concentration: The recommended freezing concentration for human PBMC is 10 × 106 to 50 × 106 cells/mL.18 We tested cryopreservation at density below 10 × 106/mL by freezing cells at a variety of concentrations between 2 × 106 and 10 × 106 cells/mL. There was no statistically significant difference in post-thaw recovery based on concentration (Figure 2A). Therefore, it is reasonable to choose from a range of concentrations based on downstream applications without risking reduced viability. Because the samples were a mix of healthy volunteer (HV) and transplant patients pretransplant and posttransplant, we also compared recovery between groups. There was no statistically significant difference in recovery based on patient status (Figure 2B).
-
(G)
Freezing rate: There is substantial data that the rate of freezing effects both cell viability and functionality. The quoted “optimal” rate of freezing is approximately 1°C/minute.19 Published data indicate very little difference in survival with media at 4°C or RT,20 indicating that cooling media before freezing is not necessary to prevent DMSO toxicity. Therefore, choosing a freezing media temperature on the basis of other experimental requirements is acceptable. As cells freeze, intracellular water is able to exit the cells due to osmotic pressure gradients. When cooled too quickly, water remaining inside the cells freezes, forming crystals that damage the cells. Conversely, cooling too slowly prolongs contact with toxic effects of DMSO.
Electronic controlled rate freezers can precisely regulate the cooling rate during freezing. However, the equipment is expensive and requires a substantial volume of liquid nitrogen to operate. Mr. Frosty is a portable nonelectronic device that aims at controlled rate freezing. The outer container of Mr. Frosty is filled with isopropanol as a mechanism to control the temperature incline in the inner container where cryovials are housed. The isopropanol should be changed every five uses. The CoolCell cell-freezing devices can achieve 1°C/minute freezing rate in a −80°C freezer without additional electronics or solutions. We found that these devices, where properly used, performed comparably to an electronic controlled rate freezer (Figure 3).
-
(H)
Storage temperature below −80°C. Long-term storage in liquid nitrogen is essential to avoid significant loss of functional activity in human PBMC. There remains residual metabolic activity even at −80°C, because molecular movement does not fully stop until cells reach the glass transition of water, around −130°C.21 Storage either in the liquid phase or vapor phase of a liquid nitrogen freezing system is sufficient to fully freeze any water present and eliminate metabolic activity.
-
(I)
Temperature increases after cells are frozen. Once transferred to liquid nitrogen, vials and cells must not be allowed to warm. Boxes should be kept in the vapor phase of liquid N2 during any transfers. Cryovials should be handled from the lids rather than on the sides of tubes to avoid any potential warming. Should cells need to be transported long distances, this should be done in liquid nitrogen—even the temperature of dry ice (approximately −75°C) for shipping will warm the cells and thus decrease viable recovery.
-
(J)
Freeze-thaw cycles. If at all possible, PBMC should be frozen in aliquots small enough for single use. If not, refreeze into aliquots of such size immediately. Do not thaw and refreeze more than once. If an aliquot is 10 × 106 cells or smaller, use the whole aliquot. In our experience, thawing and refreezing once may be acceptable for preserving the viability of the majority of cells.
-
(K)
Cell resting conditions: it has been reported pre-culture PBMC at high density of 10 × 106/mL dramatically enhanced the responsiveness of the cells in later assays.10 The study found that high-density preculture allowed autologous monocytes to prime the T cells by capping T-cell receptors and increasing basal tyrosine phosphorylation. Although this study did not compare PBMC rest at high and low density after thaw, it is conceivable that resting culture density can affect the responsiveness of the T cells. Make sure to choose the appropriate TC container for the cell numbers. For example, 2 mL of cells in a 10-mL flask risks drying out, and cell death. Or putting cells in one well of a 6-well plate while the others are empty may risk evaporation; put media in other wells of a plate to minimize evaporation risk.
FIGURE 2.
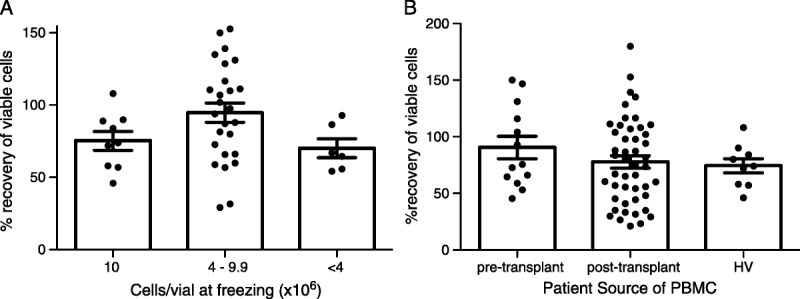
Recovery of viable human PBMC frozen at different concentrations. PBMC from HVs and transplant recipients were isolated and frozen at the indicated concentrations by the University of Pennsylvania Human Immunology core. Viable cells were counted before freezing. Cells were frozen in a Mr. Frosty at −80°C and transferred to liquid nitrogen 1 to 2 days later. The number of viable cells post-thaw was divided by the number of viable cells prefreeze to determine % recovery (n = 41 samples thawed at 6 independent times). Patients were divided on the basis of (A) number of cells frozen and (B) source of sample. Differences in recovery were analyzed using an unpaired Student’s t test. For each comparison between groups, P was greater than 0.05. HV, healthy volunteers.
FIGURE 3.
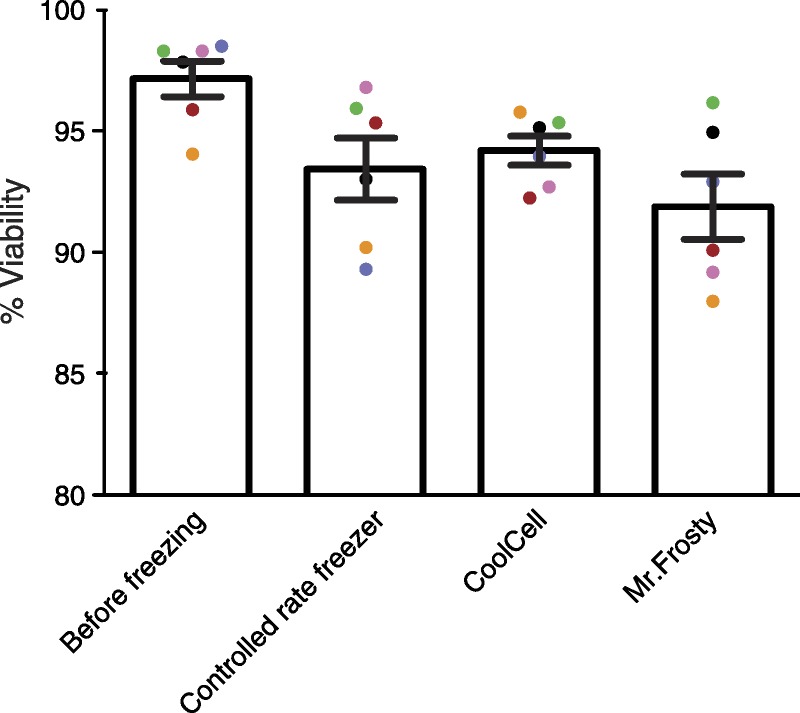
Comparison of cell viability of human PBMC cryopreserved with different devices. PBMC from healthy volunteers (n = 6, color-coded) were isolated and frozen using the indicated methods. Cell viability before freezing was measured for the baseline. Cells frozen in a controlled rate freezer were moved to a liquid nitrogen dewar upon completion of freezing cycle, whereas cells frozen in CoolCell and Mr. Frosty were transferred from −80°C to liquid nitrogen the following day. Ten days after transfer to liquid nitrogen, cells were thawed and viability measured. Results represent observations in at least 2 independent experiments.
Summary
This standard operating procedure provides information on standardization of blood collection, PBMC isolation, cryopreservation and thawing. Many variables affect cell viability, recovery, and functionality. Alternatives provided in this standard operating procedure will allow for selection of an optimal protocol for the needs of each specific study. We recommend choosing 1 method for preparation of all samples to minimize experimental variability.
ACKNOWLEDGMENTS
Members of the Virtual Global Transplant Laboratory steering committee include: Anita Chong (University of Chicago, USA), Edward Geissler (University of Regensburg, Germany), Megan Levings (University of British Columbia, Canada), Jonathan Maltzman (Stanford University, USA), Birgit Sawitzki (Free University of Berlin, Germany), Qizhi Tang (University of California San Francisco, USA), Stefan Tullius (Harvard University, USA).
The authors thank Nina Luning Prak (University of Pennsylvania Human Immunology Core), UCSF Transplantation Research Lab immune monitoring core, Morgan Reuter (Postdoctoral fellow, Betts lab, University of Pennsylvania), and Audrey Lau (Instructor, Stanford University).
Footnotes
Published online 18 August 2016.
L.E.H. was funded in part by NIH (T32 DK007357-31). Publication costs for this work were provided by the basic science committee of the Transplantation Society.
The authors declare no conflicts of interest.
L.E.H., K.L., Q.T., and J.S.M. contributed equally to this work.
L.E.H. participated in performance of research, writing and editing of the article. K.L. participated in performance of research, writing, and editing of the article. Q.T. participated in writing and editing of the article. J.S.M. participated in writing and editing of the article.
Contributor Information
Collaborators: on behalf of the Virtual Global Transplant Laboratory Steering Committee
REFERENCES
- 1.Kumar P, Satchidanandam V. Ethyleneglycol-bis-(beta-aminoethylether) tetraacetate as a blood anticoagulant: preservation of antigen-presenting cell function and antigen-specific proliferative response of peripheral blood mononuclear cells from stored blood. Clin Diagn Lab Immunol. 2000;7:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bull M, Lee D, Stucky J, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokota M, Tatsumi N, Nathalang O, et al. Effects of heparin on polymerase chain reaction for blood white cells. J Clin Lab Anal. 1999;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng AA, Lee BT, Teo TS, et al. Optimal cellular preservation for high dimensional flow cytometric analysis of multicentre trials. J Immunol Methods. 2012;385:79–89. [DOI] [PubMed] [Google Scholar]
- 5.Kreher CR, Dittrich MT, Guerkov R, et al. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79–93. [DOI] [PubMed] [Google Scholar]
- 6.Mallone R, Mannering SI, Brooks-Worrell BM, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen RE, Sinclair E, Emu B, et al. Loss of T cell responses following long-term cryopreservation. J Immunol Methods. 2007;326:93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg A, Song L-Y, Wilkening C, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009;16:1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:C125–C142. [DOI] [PubMed] [Google Scholar]
- 10.Römer PS, Berr S, Avota E, et al. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011;118:6772–6782. [DOI] [PubMed] [Google Scholar]
- 11.Ernst D, Ballance L, Calam R, et al. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture; Approved Standard. 6th Edition Clinical and Laboratory Standards Institute; 27:H3–A6. [Google Scholar]
- 12.Isolation of mononuclear cells Methodology and applications. GE Healthcare Life Sciences. 18-1152-69 AE. [Google Scholar]
- 13.Chan LL, Laverty DJ, Smith T, et al. Accurate measurement of peripheral blood mononuclear cell concentration using image cytometry to eliminate RBC-induced counting error. J Immunol Methods. 2013;388:25–32. [DOI] [PubMed] [Google Scholar]
- 14.Davis R, Hseuh R. Heat inactivation of fetal bovine serum; AfCS Procedure Protocols. 2002. [Google Scholar]
- 15.Reimann KA, Chernoff M, Wilkening CL, et al. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. The ACTG Immunology Advanced Technology Laboratories. Clin Diagn Lab Immunol. 2000;7:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hokland P, Heron I. The isopaque-Ficoll method re-evaluated: selective loss of autologous rosette-forming lymphocytes during isolation of mononuclear cells from human peripheral blood. Scand J Immunol. 1980;11:353–356. [DOI] [PubMed] [Google Scholar]
- 17.Kloverpris H, Fomsgaard A, Handley A, et al. Dimethyl sulfoxide (DMSO) exposure to human peripheral blood mononuclear cells (PBMCs) abolish T cell responses only in high concentrations and following coincubation for more than two hours. J Immunol Methods. 2010;356:70–78. [DOI] [PubMed] [Google Scholar]
- 18.Simione F, Brown E, (Eds.). ATCC preservation methods: freezing and freeze-drying. 2nd ed. Rockville, MD: University of Michigan; 1991. [Google Scholar]
- 19.Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007;368:39–57. [DOI] [PubMed] [Google Scholar]
- 20.Nazarpour R, Zabihi E, Alijanpour E, et al. Optimization of human peripheral blood mononuclear cells (PBMCs) cryopreservation. Int J Mol Cell Med. 2012;1:88–93. [PMC free article] [PubMed] [Google Scholar]
- 21.Johari GP, Hallbrucker A, Mayer E. The glass-liquid transition of hyperquenched water. Nature. 1987;330:552–553. [Google Scholar]


