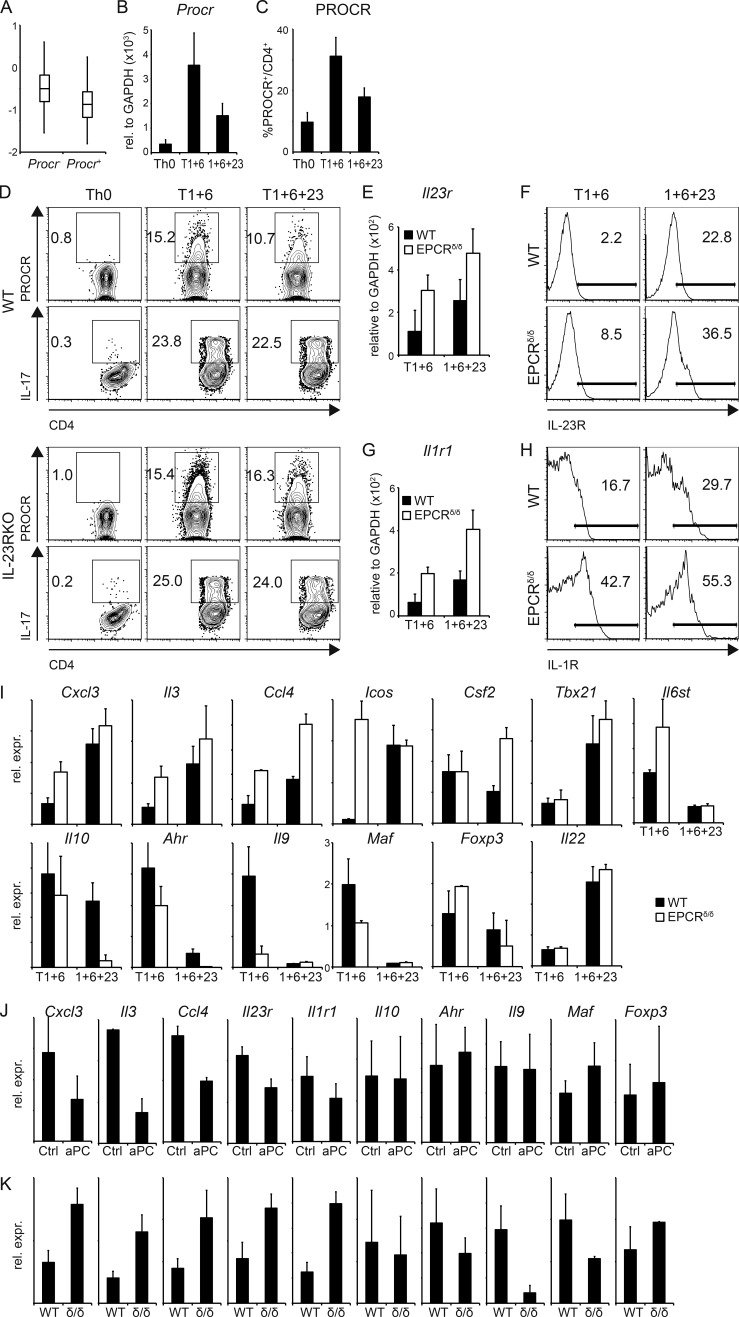
Figure 4.
PROCR regulates genes of the proinflammatory module in Th17 cells. (A) Th17 cells differentiated in vitro with TGF-β1 + IL-6 were analyzed using single-cell RNA-seq (Gaublomme et al., 2015), and Procr expression in these cells was correlated with the pathogenic Th17 signature (Lee et al., 2012). P = 1.18 × 10−3 (Wilcoxon ranks test comparing signature scores of Procr+ vs. Procr – cells). (B and C) Naive CD4+ T cells from WT mice were differentiated into Th17 cells by anti-CD3/anti-CD28 stimulation in the presence of TGF-β1 + IL-6 (T1+6), IL-1β + IL-6 + IL-23 (1+6+23), or no cytokines as control (Th0). PROCR expression was determined by quantitative RT-PCR (B) and flow cytometry (C) 72 h later. Representative plots of three independent experiments are shown. (D) WT or IL-23R KO Th17 cells generated by anti-CD3/anti-CD28 stimulation in the presence of TGF-β1 + IL-6 (T1+6), TGF-β1 + IL-6 + IL-23 (T1+6+23), or no cytokines as control were analyzed for PROCR expression and IL-17 secretion by flow cytometry 72 h later. Representative plots of three independent experiments are shown. (E–H) Naive CD4+ T cells from WT and EPCR δ/δ mice were differentiated into Th17 cells as in B. IL-23R (E and F) and IL-1R1 (G and H) expression was determined by quantitative RT-PCR (E and G; mean ± SD of three independent experiments) and flow cytometry (F and H) 96 h later. (I) Quantitative RT-PCR analysis of pathogenic and nonpathogenic signature genes in WT (filled bars) and EPCR δ/δ (open bars) Th17 cells at 96 h. (J and K) Quantitative RT-PCR analysis of pathogenic and nonpathogenic signature genes (J) in Th17 cells differentiated with TGF-β1 + IL-6 with or without (Ctl) aPC and (K) in Th17 cells from WT or EPCRδ/δ (δ/δ) mice after 3 d of culture. (I–K) Bar graphs represent mean ± SEM of at least three independent experiments.