Fig. 1.
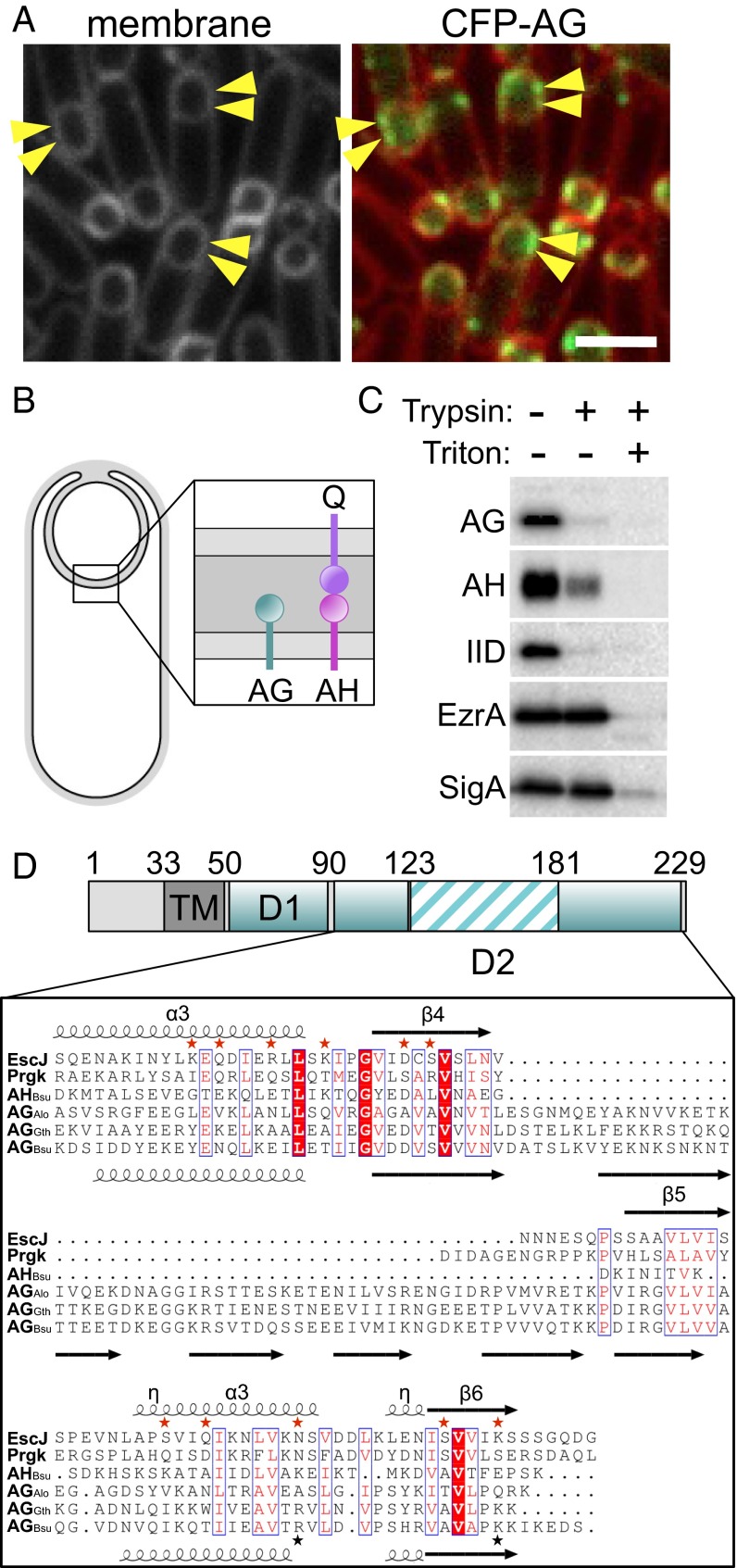
The extracellular domain of AG has remote homology to PrgK/EscJ proteins in type III secretion systems. (A) CFP-SpoIIIAG (CFP-AG, false-colored green) localizes as discrete foci (arrowheads) in the mother cell membranes that surround the forespore. CFP-AG displays weak localization at the second potential polar division site, but the relevance of this localization remains unclear. CFP-AG and membranes (stained with the fluorescent probe TMA-DPH) were visualized by fluorescence microscopy at hour 2 of sporulation. (Scale bar, 2 μm.) (B) Schematic diagram showing the localization and topology of AG, SpoIIIAH (AH), and SpoIIQ (Q) during sporulation. (C) The extracellular domains of AG, AH, and SpoIID (IID) are extracytoplasmic. Immunoblot of a protease susceptibility assay in which sporulating cells were protoplasted and treated with trypsin with or without Triton X-100. A soluble transcription factor (SigA) and a membrane-anchored cytoplasmic protein (EzrA) were inaccessible to trypsin. (D) Domain structure of AG showing the transmembrane segment (TM) and the extracytoplasmic D1 and D2 domains. Numbering refers to the B. subtilis AG sequence. The sequences of AG from B. subtilis (AGBsu), G. thermodenitrificans NG-80 (AGGth), and A. longum (AGAlo) were aligned with S. typhimurium SPI‐1 and PrgK and E. coli EPEC LEE EscJ. Conserved residues are in red boxes; similar residues are shown by red letters boxed in blue. The secondary structures of EscJ and the ones predicted for AGBsu are indicated above and below the sequence alignment, respectively. Arrows indicate β-strands; α, α-helix; η, 310-helices. Residues at the oligomeric interface of EscJ protomers are indicated by red stars. Predicted AGBsu interface residues important for ring formation and sporulation are indicated by with black stars.