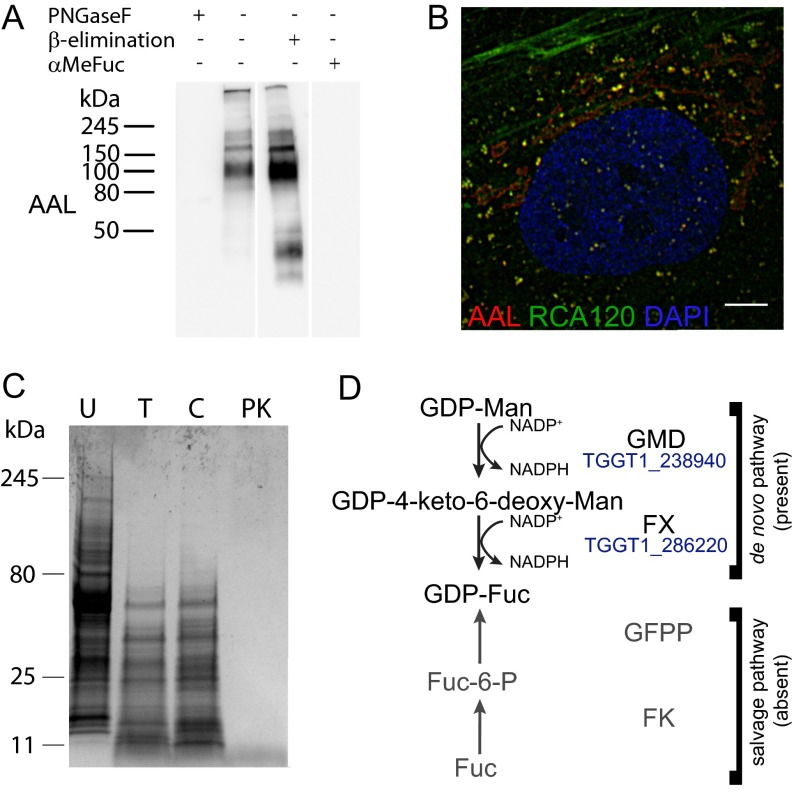
Fig. S2.
(A) AAL binds to N-glycans in the host cell because PNGase F inhibits AAL binding to the HFF lysate. (B) AAL-positive glycans are found in the ER and secretory vesicles of the host cells (deconvolution fluorescence microscopy). (Scale bar: 5 μm.) (C) Coomassie staining in a protease experiment on T. gondii tachyzoite cell lysate. C, chymotrypsin; PK, proteinase K; T, trypsin; U, untreated. (D) The de novo GDP-fucose biosynthesis pathway, composed of GDP-mannose-4,6-dehydratase (GMD, EC 4.2.1.47) and GDP-fucose synthase (FX, EC 1.1.1.271), is present in the predicted T. gondii proteome. No salvage pathway (shown in gray) can be identified: fucokinase (FK, EC 2.7.1.52) and GDP-fucose pyrophosphorylase (GFPP, EC 2.7.7.30) homologs were searched using Homo sapiens FK and GFPP or the bifunctional Leishmania major and Arabidopsis thaliana enzymes as templates. No homologs for the biosynthesis of GDP-Rhamnose or UDP/dTDP-Rhamnose could be found; dTDP/UDG-glucose-4,6-dehydratase (dTDG/UDG, EC 4.2.1.46/47), dTDP/UDP-Rhamnose synthase (RX, EC1.1.1.133), the trifunctional GDP-Rhamnose synthase (RHM1), and GDP-6-deoxy-mannose reductase (RMD, EC 1.1.1.281) from A. thaliana, Trypanosoma cruzi, Escherichia coli, or Pseudomonas aeruginosa were used as templates.