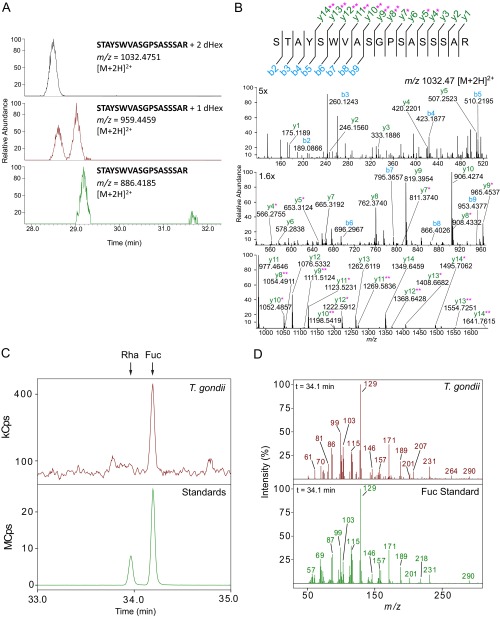
Fig. S4.
AAL binds proteins modified by one or more O-Fuc on Ser/Thr residues, as verified by neutral loss of dHex, MS/MS fragmentation, and GC-MS monosaccharide analysis. (A) XICs obtained from LC-MS spectra of a glycopeptide from TGGT1_285190 show neutral loss of two to one dHex(s) and in smaller amounts of one to zero dHex. (B) MS/MS spectrum for the glycoform with two dHexs, m/z 1032.47 [M+2H]2+. The y and b series are marked in green and blue, respectively. Fragment ions plus one or two dHexs are marked with one and two pink asterisks, respectively. (C) GC-MS extracted ion chromatograms for the T. gondii sample and the standards mixture. The matched retention time compared with the standard indicates that fucose is the only dHex present after reductive β-elimination release of sugars from AAL-enriched T. gondii proteins. XICs were generated from the ions (129 and 171 m/z), which are abundant in the EI spectra from dHex alditol acetates. XICs from 33 to 35 min are shown; the arrows indicate the rhamnose (Rha) and fucose (Fuc) retention times. (D) GC-MS EI spectra of the alditol acetates from the T. gondii sample and Fuc alditol acetate standard. The retention time (34.1 min) from the XIC shown in C was used to generate the spectrum for each. Both the ions pattern and retention time match the Fuc standard and not that of Rha.