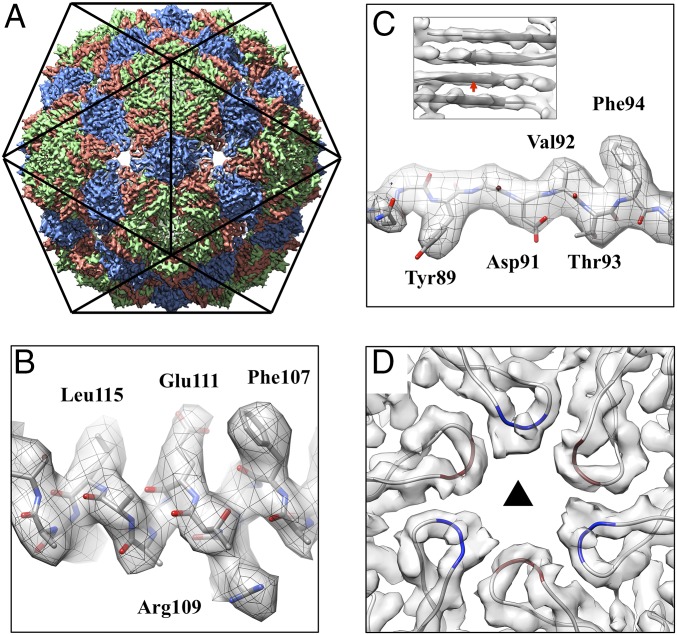
Fig. 1.
Icosahedral reconstruction of the Qβ coat protein shell at 3.7-Å resolution. (A) The map is oriented with the twofold axis pointing out of the figure. Coat protein conformers A, B, and C are colored salmon, green, and blue, respectively. (B) The α-helix density of the coat protein showing α-helical pitches and bulky amino acid side-chains. (C) The bulky side-chains in the β-strand. (Inset) Separation of four β-strands in a β-sheet formed within coat protein dimers, with the red arrow indicating the location and viewing angle for the C. (D) The cryo-EM densities and models of the FG-loops, located at the threefold axis, with residues 76–79 for conformers A and C colored red and blue, respectively. The black triangle indicates the threefold axis.