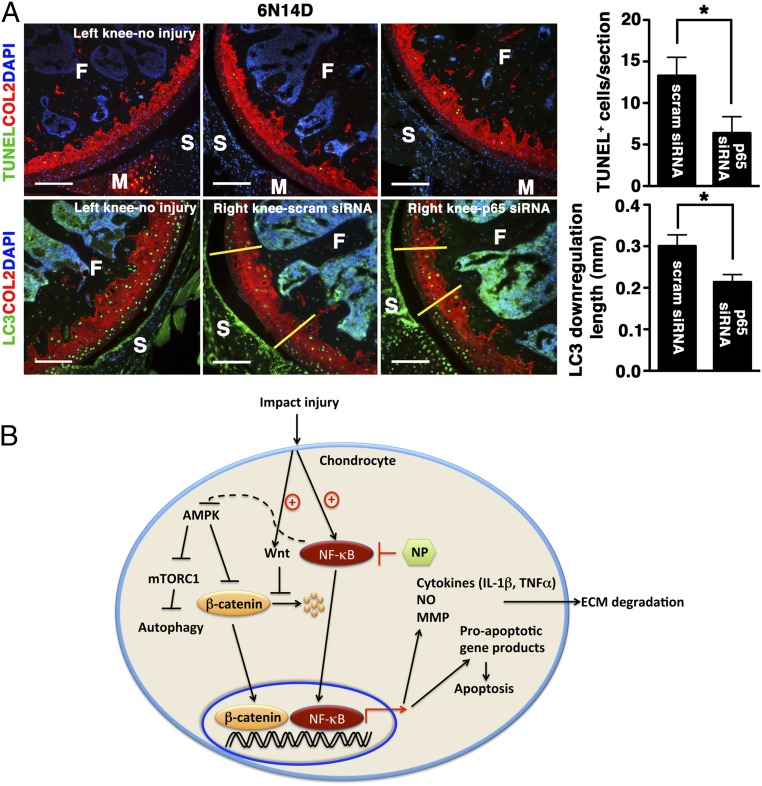
Fig. 7.
Extended effects of p5RHH-p65 siRNA NP on cartilage. (A) Mice were subjected to compression injury at 6 N and injected immediately i.a. with 0.1 μg of p5RHH-p65 or p5RHH-scrambled (scram) siRNA NP; the dose was repeated at 24 h and at 48 h (for a total of three doses). On day 14, knees were harvested and analyzed for TUNEL+ cells (green, Upper) and length of LC3 down-regulation (green: Lower, between yellow demarcation lines). Note that the number of TUNEL+ cells decreased over time. Values represent mean ± SEM; n = 4 mice per treatment (Tx) group. COL2 (red), type II collagen; F, femur; M, meniscus; S, synovium. DAPI (blue) stains nuclei. (Scale bars, 100 μm.) *P < 0.05. (B) Graphical summary. Impact injury activates NF-κB–signaling pathways in chondrocytes, leading to expression of inflammatory cytokines that promote ECM degradation and expression of proapoptotic gene products that induce apoptosis. Administration of p5RHH–NF-κB siRNA NP blocks these downstream effects. p5RHH–NF-κB siRNA nanotherapy also potentially preserves chondrocyte/cartilage homeostasis through AMPK activation that, in turn, blocks mTORC1, thus enhancing autophagy. Impact injury likely stimulates Wnt signaling, as evidenced by increased β-catenin level and nuclear translocation. p5RHH–NF-κB siRNA nanotherapy suppresses β-catenin expression, thus reducing the expression of catabolic genes (such as metalloproteinases).