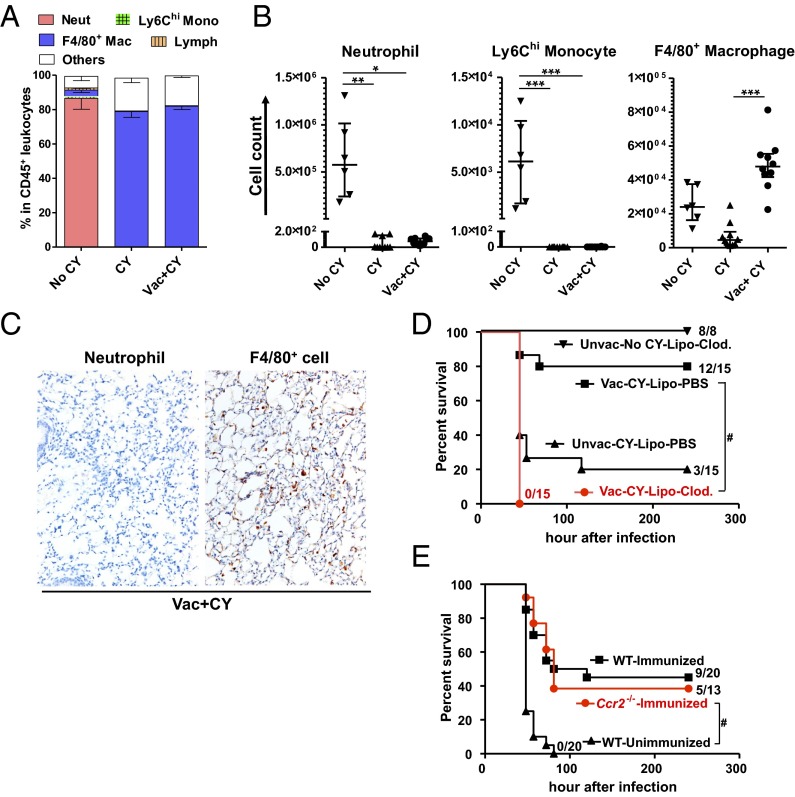
Fig. 2.
Macrophages are required for vaccine-induced protection during chemotherapy. Quantitation of immune cell types in BALF during infection expressed as the fraction in total CD45+ leukocytes recovered from the total BALF per mouse (A) or by absolute number in the BALF (B). Mono, monocyte; Neut, neutrophil; Vac, PAO1ΔaroA-immunized; Vac+CY, immunized plus CY-treated. BALF was obtained from untreated mice or CY-treated mice ± prior vaccination 30 h after P. aeruginosa challenge (n = 6–10 per group; 6.8 × 106 cfu for untreated mice and 68 cfu for CY-treated mice). Each symbol represents one mouse, and error bars represent median with interquartile ranges. *P < 0.05, **P < 0.01, and ***P < 0.001 by the Kruskal–Wallis test, followed by Dunn’s multiple comparisons test. (C) IHC stain for neutrophils (negative) or F4/80+ cells (brown) in lung parenchyma of immunized, CY-treated mice 30 h postinfection (69 cfu). A positive neutrophil stain performed side by side with these samples is shown in Fig. 1B. Representative data are shown from five mice. (Magnification: 20×). (D) Survival of immunized, CY-treated mice given either i.n. liposomal clodronate (lipo-Clod.) or lipo-PBS 24 h before challenge (65 cfu; no. alive/total as indicated). Unimmunized, CY-treated mice with i.n. lipo-PBS before challenge (65 cfu) or unimmunized, CY-untreated normal mice with i.n. lipo-Clod. before challenge (5.8 × 105 cfu, performed in an independent experiment from the other groups) were controls. Survival was monitored twice daily starting 24 h postinfection. #P < 0.0001 by log-rank test. Unvac, unvaccinated. (E) Survival of CCR2-deficient mice that were immunized, CY-treated, and challenged (60–69 cfu; no. alive/total as indicated). Immunized or unimmunized wild-type mice that were challenged following CY treatment were controls. Data combined from two independent experiments are shown. #P < 0.0001 by log-rank test.