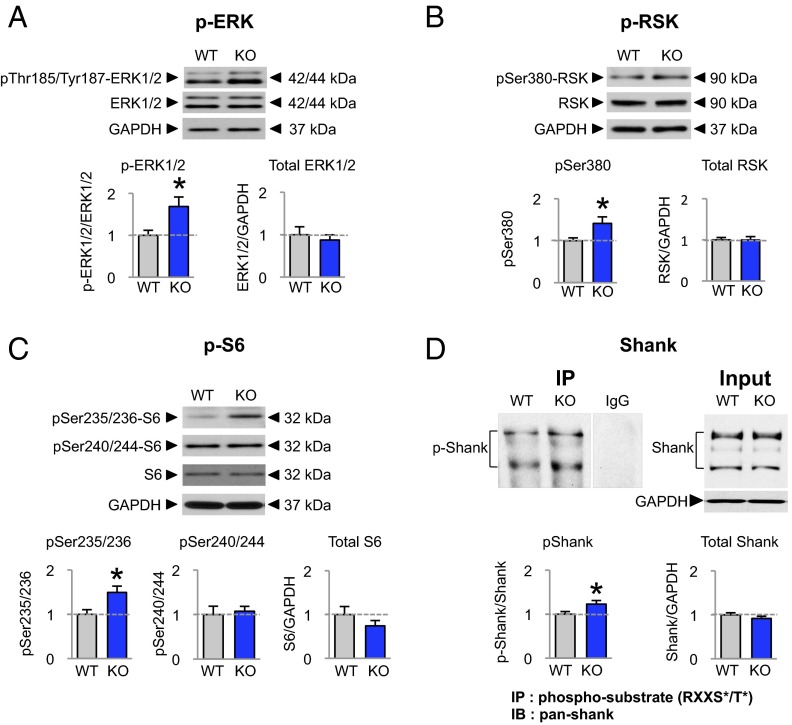
Fig. 3.
ERK/RSK/S6 signaling and Shank phosphorylation are elevated at cortical synapses of Fmr1 KO mice. (A–C) Representative Western blots of synaptosomes from the neocortex of WT and Fmr1 KO mice and summary data showing relative abundance of phosphorylated and total ERK, RSK, and S6. Phosphorylation (but not total protein) was elevated for (A) p-ERK (WT, n = 12; KO, n = 18), (B) p-RSK (WT, n = 8; KO, n = 15), and (C) p-S6 at Ser235/236 (WT, n = 9; KO, n = 17), but not at Ser240/244 (WT, n = 10; KO, n = 16). (D) Phosphorylation of the RSK target Shank is increased in the synaptosomal fraction from neocortex of Fmr1 KO mice. Phosphorylated proteins were immunoprecipitated from WT and KO synaptosomes by means of an antibody recognizing the RSK phospho-motif (phospho-RXXS*/T*). Western blots using an antibody recognizing all forms of Shank were used on the immunoprecipitate (IP) and input of synaptosomal proteins of the WT and Fmr1 KO mice. There was no detectable change in the levels of Shank in the input between WT and KO but there was significant increase in the amount of Shank immunoprecipitated by the phospho-specific antibody, indicating elevated Shank phosphorylation at the RSK motif (WT, n = 17; KO, n = 15). Data represent mean ± SEM; *P < 0.05.