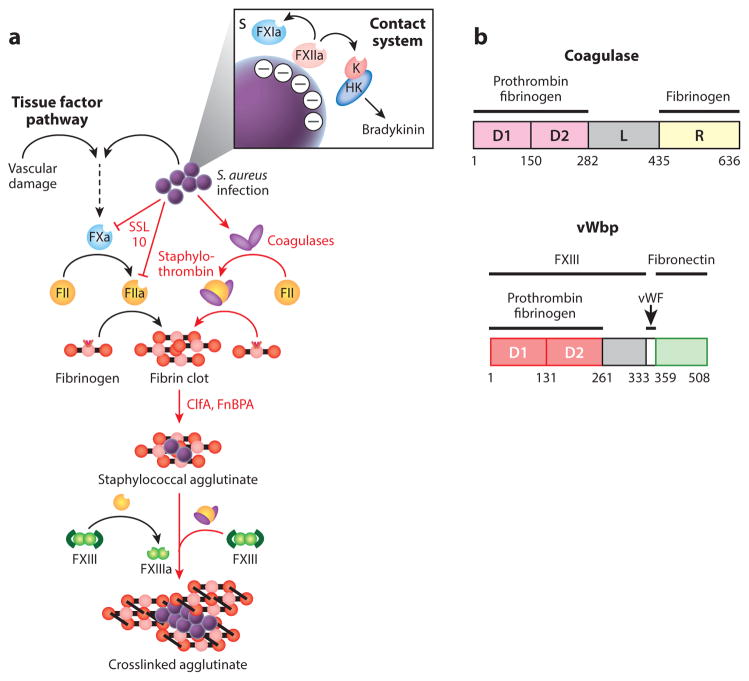
Figure 2.
Agglutination and Staphylococcus aureus interference with host hemostasis. (a) Vascular damage and tissue factor or contact activation and bradykinin are pathways for hemostasis—a cascade of proteolytic activation for the zymogens of clotting factors culminating in the conversion of prothrombin (FII) to thrombin (FIIa), which cleaves fibrinopeptides A and B from fibrinogen, thereby triggering self-assembly of fibrin cables either to stem blood loss from injured vasculature or to immobilize pathogens that have activated the contact system. S. aureus bypasses the host hemostasis pathways to activate FII/prothrombin via the secretion of coagulases (Coa and vWbp), thus yielding staphylothrombin and, via nonproteolytic activation of fibrin-stabilizing factor (FXIII), the transglutaminase responsible for crosslinking fibrin cables. ClfA, an agglutinin of the surface of S. aureus, captures staphylothrombin-derived fibrin cables, thereby agglutinating staphylococci into large aggregates of bacteria enclosed by a fibrin shield. (b) A diagram illustrating the primary translational products of Coa and vWbp from S. aureus strain Newman; the catalytic D1 and D2 domains are shown in light and dark red boxes, and the R domain of coagulase with repeats is shown in yellow. The function of the linker domain (L) is not known. The number of repeats in the R region of coagulase varies between strains. The binding sites for prothrombin, fibrinogen, FXIII, and fibronectin are delineated. Abbreviation: vWF, von Willebrand factor.