Summary
The effect of adding a hydrocolloid on the structural, mechanical and barrier properties of zein-based blend films is evaluated. Zein-oleic acid blend film with added xanthan gum (Z-OA-XG) showed higher water solubility (13.09%) and opacity (8.49 AU/mm) than zein-oleic acid (Z-OA) film (10.80% and 5.19 AU/mm, respectively). Furthermore, Z-OA film had greater flexibility with lower Young’s Modulus (YM=5.02 MPa) and higher elongation at break (η=10.62%); nonetheless, it was less resistant to tension (tensile strength σ=8.5 MPa) than Z-OA-XG film, which showed YM, η and σ of 6.38 MPa, 6.66% and 10.485 MPa, respectively. Both films had glossy and homogeneous structure with comparable water vapour and oxygen barrier properties around 4.39·10–11 and 1.82·10–13 g/(Pa·s·m), respectively. Based on that, xanthan gum structure influenced mainly mechanical and light barrier properties of zein-oleic acid blend films.
Key words: blend film, biodegradable material, permeability, microstructure, tensile properties
Introduction
Packaging is essential for food transport and distribution, enabling quality preservation and protection against external chemical, physical and microbiological contamination (1–3). Nowadays, plastic materials are the most frequently used food packaging due to their thermostability, flexibility, lightness and low price (3–5); nonetheless, as a result of the global environmental concern, new biodegradable materials, mainly films and coatings, have been developed in order to reduce plastic packaging usage (6–8). In comparison with plastics, biodegradable films and coatings are quickly degraded by the action of microorganisms as a consequence of their natural origin (3, 8).
In general, biodegradable materials are prepared from biopolymers such as proteins, carbohydrates or lipids. For instance, zein, the main corn protein, is one of the materials that have been used to produce biodegradable films and coatings for food and pharmaceutical applications (8–10). Zein-based materials have glossy appearance and low water solubility, they are tough, greaseproof, hydrophobic and resistant to microbial attack (8, 11), although brittle (12, 13).
Several studies have demonstrated that preparing biodegradable materials from two or more biopolymers, known as composites or blends, allows for improving their functional properties compared to single biopolymer films (5, 6, 14). Some composite and blend biodegradable materials that have recently been reported are: (i) cassava starch-based films with clay nanoparticles (3), (ii) pea starch and peanut protein isolate blend films (5), (iii) nanocrystalline cellulose-reinforced chitosan-based nanocomposite films (6), and (iv) zein-wax composite and zein-fatty acid blend films (12). These composite and blend materials showed better homogeneity or improved mechanical and water vapour barrier properties than the single biopolymer ones.
Cuq et al. (15) and Wang and Padua (16) reported that zein-based films blended with oleic acid had better water vapour barrier properties and flexibility. Barbosa De Almeida et al. (17) reported the improvement of zein-oleic acid film homogeneity when different concentrations of xanthan gum were added; nevertheless, their effect on the film’s functional properties was not evaluated. Following this idea, the aim of the current study is to evaluate the effect of adding xanthan gum on the structural and functional properties of zein-oleic acid blend films.
Materials and Methods
Materials
Zein from maize (Sigma-Aldrich Brazil. Ltda., Săo Paulo, Brazil), 99.5% ethanol (Synth, Săo Paulo, Brazil), glycerol (Dinâmica, Diadema, Brazil), oleic acid (Synth), xanthan gum (ADM, Chicago, IL, USA) and Emustab emulsifier (Duas Rodas, Jaraguá do Sul, Brazil) were used for preparing blend films. Calcium nitrate (Dinâmica) and sodium bromide salts (Synth) were used for water vapour permeability tests and sample conditioning, respectively.
Film composition and preparation
Zein-based films blended with oleic acid (Z-OA) and zein-based films blended with oleic acid and xanthan gum (Z-OA-XG) were prepared by adding 20% (by mass per volume) zein to 95% aqueous ethanol during 5 min of mechanical stirring at (65±0.5) °C. Glycerol, emulsifier and oleic acid were added to the solution at 10, 5 and 70% (by mass), respectively; then 0.05% (by mass per volume) xanthan gum was added to form the Z-OA-XG solution and then the Z-OA and Z-OA-XG solutions were mechanically stirred for 10 min. A volume of 50 mL of solution was poured onto a rectangular glass plate and air-dried overnight at room conditions. Films were peeled off and conditioned according to ASTM D618-13 (18) at 57.6% relative humidity (RH) and (24±2) °C for at least 40 h prior to analyses.
Film thickness
Thickness was measured with a digital micrometer (model P54; Digimess Instruments Ltd., Derby, UK) with 0.001 mm precision. Five thickness measurements were taken, one at the centre and four around the perimeter. The average thickness value was used in further calculations.
Light permeability (opacity)
Opacity was assessed at 600 nm in a spectrophotometer (model SP-220; Bioespectro Equipar, Curitiba, PR, Brazil). Film samples were cut into 9 mm×43 mm rectangular shapes and placed in the internal side of the spectrophotometer cell (14, 19). Six specimens of each film were tested and opacity was calculated according to the following equation:
where x is the average film thickness in mm and A600 nm is the absorbance at 600 nm.
Water solubility
Film water solubility was defined as the content of dried matter solubilized after 24 h of immersion in water. Films were cut into 2-cm (diameter) disks and dried in oven at (105±2) °C for 24 h. Samples were weighed (initial mass, mi) and immersed into 50 mL of distilled water at (27±2) °C for 24 h under agitation in an orbital shaker (model MA-410; Marconi, Piracicaba, SP, Brazil) at 76 rpm. After 24 h of immersion, the samples were taken out and dried (final mass, mf) under the same conditions mentioned before, to determine the mass of the dried matter that was not solubilized in the water (14, 20). Three specimens of each film were tested and water solubility was calculated based on the following equation:
Water vapour permeability
Water vapour permeability (WVP) of the films was measured gravimetrically according to the ASTM E96/96M-14 standard, desiccant method (21). Test cells were covered and sealed by the film samples and placed in a controlled chamber (desiccator) maintained at RH=51% by a saturated solution of calcium nitrate. Silica gel activated at 200 °C was used to maintain RH=0% inside the test cells. Desiccator was stored at (24±2) °C. Three specimens of each film were tested and WVP was calculated according to the following equation:
where S is the saturation of vapour pressure at test temperature (2985 Pa at 24 °C), R1 is RH in the test desiccator expressed as a fraction, R2 is RH inside the test cell expressed as a fraction and x is the average film thickness. WVT is the water vapour transmission, calculated as follows:
where mg is the mass change (gain) of the test cell, t is the time needed for the mass change and a is the test area (12.57 cm2). The ratio of mg /t was obtained from the slope of the linear portion of the plot of mg vs. t.
Oxygen permeability
The oxygen transmission rate (OTR) was measured according to the ASTM F1927-14 standard (22), using an oxygen permeation instrument (OX-TRAN® model 2/20; Mocon, Minneapolis, MN, USA). OTR of the film was determined under control conditions (23 °C and RH=0%). The film was placed between two sides of the test chamber, one side was exposed to carrier gas containing 98% N2 and 2% H2 while the other side was exposed to test gas (pure O2). The sensor monitored the exit port of the carrier gas side measuring the amount of present oxygen. OTR was calculated according to the following equation:
where V(O2) is the measured volume of oxygen, t is the time required for reaching the stationary state and a is the transfer film area (100 cm2). Oxygen permeability (OP) was calculated as follows:
where x is the film average thickness and p is the partial oxygen pressure.
Mechanical test
Mechanical properties were evaluated according to the ASTM D882-12 standard (23). Elongation at break (η), tensile strength (σ) and Young’s modulus (YM) were tested with a texture analyzer (TA.XT Plus; Stable Microsystems Ltd., Godalming, Surrey, UK) using the Exponent software and the A/TG probe (Stable Microsystems Ltd.). Test films were cut with a guillotine according to ASTM D6287-09 standard (24) into rectangular strips 100 mm long and 15 mm wide. Thickness was measured in five points along the sample in order to assure thickness uniformity. Initial grip separation and crosshead speed were set at 50 mm and 500 mm/min, respectively. Stress and strain were recorded during the extension of the strips and minimum 5 specimens were analyzed.
Microstructure
Film superficial and cross-section microstructure were observed under high vacuum and constant temperature using a scanning electron microscope (model EVO LS15; Carl Zeiss, Jena, Germany) equipped with secondary electron detector. Film samples were covered with gold and images were taken at 15–20 kV with magnification of 1000 and 5000×. Cross-section images were obtained by cryogenic fracture; immersing the samples into liquid nitrogen for 2 min. Film samples were fixed to the microscope stubs using a conductive carbon double-sided tape.
Statistical analysis
Statistical analysis was accomplished with Statgraphics Centurion, v. 16.1 (Statpoint Technologies, Inc., Warrenton, VA, USA). An analysis of variance and a Tukey’s multiple comparison test were performed to detect significant differences between Z-OA and Z-OA-XG film properties with 5% significance level.
Results and Discussion
Z-OA and Z-OA-XG blend films were homogeneous and smooth, without visible pores and without greasy touch perception; film components were visibly well integrated in the matrix. Z-OA-XG blend film had glossy appearance and brittle touch perception probably due to the packed and arranged structure of the xanthan gum polysaccharides (25).
Opacity of the zein-based blend films
Opacity is an indicator of the light barrier property of the film, representing the capacity of the film to protect the product against deterioration caused by light. The Z- -OA-XG film exhibited an increase in the light barrier property over 60% compared to the Z-OA film (Table 1). The addition of xanthan gum to zein-oleic acid blend film promoted the reduction of light transmission through the film, possibly as a consequence of the packed structure of the polysaccharide.
Table 1. Water solubility and light barrier properties of zein- -based blend films.
| Film |
x mm |
Water solubility % |
Opacity mm–1 |
|---|---|---|---|
| Z-AO | (0.062±0.008)a | (10.8±0.5)a | (5.19±0.6)a |
| Z-AO-GX | (0.062±0.011)a | (13.1±1.4)b | (8.49±0.4)b |
Values are mean±standard deviation. Different letters indicate statistically significant difference (p<0.05). x=thickness
Blend films of soya protein isolate and gelatin reported by Denavi et al. (26) exhibited lower opacity than Z-OA and Z-OA-XG blend films. Besides, the Z-OA-XG blend film of the current study showed higher light barrier than the same blend film reported by Barbosa De Almeida et al. (17). The higher opacity displayed by the Z- -OA-XG blend film in this study is probably due to the difference in the composition of both films. For instance, glycerol addition helps improving film flexibility by creating spaces in the matrix (27), although it decreases the film light barrier. Thus, the lower glycerol volume fraction in the Z-OA-XG film improved its opacity. Similarly, Barbosa De Almeida et al. (17) reported blend films prepared with 75% ethanol, while Z-OA-XG blend films here were prepared with 95% ethanol. Higher ethanol volume fraction improves zein solubilization, increasing intermolecular interactions between protein chains and between protein and other film components; hence, creating a tight film matrix that prevents light transmission (27).
Water solubility of the films
Water solubility indicates the water affinity of the film, representing its water-resistance capacity. As shown in Table 1, Z-OA-XG film displayed greater solubility than Z-OA film, possibly due to the higher polarity of the film caused by the addition of a hydrophilic compound (11, 25).
Polysaccharide-based films produced from chitosan, galactomannan or agar exhibited water solubility of over 22% (20). Similarly, the water solubility of gelatin-based films blended with 1% sunflower oil was around 80% (14). Based on that, Z-OA and Z-OA-XG films showed greater water-resistance capacity than the films mentioned above, due to the hydrophobic nature of zein as well as the high oleic acid volume fraction of the Z-OA and Z-OA-XG blend films.
Gas barrier properties of the films
The gas barrier properties of the Z-OA and Z-OA-XG blend films that were assessed were water vapour and oxygen permeability (Table 2). Water vapour permeability (WVP) refers to the barrier property of the film, indicating the degree of moisture transfer between the packaged product and the surroundings. As shown in Table 2, both blend films, Z-OA and Z-OA-XG, displayed similar WVP values. Therefore, increasing the film’s polarity by adding xanthan gum had no effect on moisture transfer, which was expected, based on previously published data (11, 28). This is probably a consequence of the low xanthan gum fraction added to Z-OA-XG film.
Table 2. Water vapour and oxygen permeability properties of zein-based blend films.
| Film |
x mm |
WVP g/(Pa·s·m) |
OP g/(Pa·s·m) |
|---|---|---|---|
| Z-AO | 0.065±0.005 | (4.4±0.7)·10–11 | (1.9±0.3)·10–13 |
| Z-AO-GX | 0.063±0.000 | (4.4±0.6)·10–11 | (1.8±0.3)·10–13 |
Values are mean±standard deviation and they were not statistically different (p<0.05). x=thickness, WVP=water vapour permeability, OP=oxygen permeability
The blend film based on konjac glucomannan, chitosan and soya protein isolate reported by Jia et al. (29) showed higher WVP (5.18·10–11 g/(Pa·s·m)) than Z-OA and Z-OA-XG blend films. Likewise, protein-based films produced from whey protein isolate (30) and blend films prepared from zein and wheat gluten (31) displayed higher WVP values (66·10–9 and 5.0·10–11 g/(Pa·s·m), respectively) in comparison with Z-OA and Z-OA-XG films. Based on that, the Z-OA and Z-OA-XG blend films exhibited a better water vapour barrier property than other biodegradable films as a consequence of their higher hydrophobicity.
Similarly to WVP, oxygen permeability (OP) indicates the oxygen transfer between the packaged product and the surroundings. This film property is of great importance when the product can suffer oxidative reactions that deteriorate its composition and integrity.
As shown in Table 2, the addition of xanthan gum to zein-oleic acid blend films had no impact on film oxygen permeability. Both films, Z-OA and Z-OA-XG, displayed similar OP values (p<0.05), likely caused by the low fraction of xanthan gum that was added.
Z-OA and Z-OA-XG blend films exhibited higher OP in comparison with polysaccharide-based films reported by Cerqueira et al. (20) and Matta et al. (32) which showed oxygen permeability around 1·10–15 and 6.3·10–17 g/(Pa·s·m), respectively. This was probably an effect of the high oleic acid concentration, which increased oxygen solubilization and its diffusion through the film (33). Furthermore, several studies have reported better oxygen barrier properties of polysaccharide-based films than protein- and lipid-based films due to the structure and polarity of the polysaccharides (34).
Mechanical properties of the films
Mechanical properties such as tensile strength (σ), elongation at break and Young’s modulus (YM) represent the toughness and elasticity (η) or brittleness of the film. Thus, as shown in Table 3, Z-OA-XG film displayed greater toughness (higher σ) than Z-OA due to the packed and arranged structure of xanthan gum; nevertheless, polysaccharide structure increased the stiffness and brittleness of Z-OA-XG blend film (higher YM and lower η, respectively).
Table 3. Mechanical properties of zein-based blend films.
| Film |
x mm |
σ MPa |
η % |
YM MPa |
|---|---|---|---|---|
| Z-AO | (0.066±0.004)a | (8.5±0.5)a | (10.6±2.3)a | (5.0±0.3)a |
| Z-AO-GX | (0.065±0.004)a | (10.5±0.6)b | (6.7±1.3)b | (6.4±0.5)b |
Values are mean±standard deviation. Different letters indicate statistically significant difference (p<0.05). x=thickness, σ=tensile strength, η=elasticity, YM=Young’s modulus
For instance, gelatin films and 10% zein-based films with 3% glycerol had higher tensile strength, around 22 and 10 MPa, respectively (35), in comparison with Z-OA and Z-OA-XG films. The lower σ of the zein-based films in this study was probably due to the higher glycerol volume fraction (10%) added to the films as well as the high oleic acid fraction. Glycerol and oleic acid are plasticizer agents that reduce the tensile strength of the films (33). Moreover, protein-based films produced from cowpea protein isolate with 10% polyethylene glycol reported by Hewage and Vithanarachchi (36) had lower tensile strength (σ=6.6 MPa) compared with Z-OA and Z-OA-XG films.
Blend film microstructure
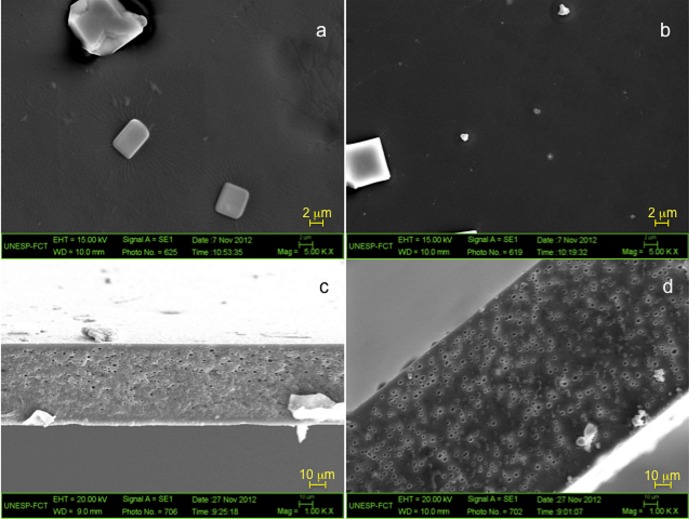
Scanning electron microscopy allows for the evaluation of the distribution and integration of film components into the matrix. Z-OA and Z-OA-XG blend films displayed homogeneous surfaces with good integration of film components (Figs. 1a and b). Film micrographs showed the presence of square-shaped materials on Z-OA and Z-OA-XG film surfaces. As both films had the same materials on the surface, we believed that these were due to contaminant substances in the reagents used for film preparation.
Fig. 1.
SEM micrographs of film surface at 5000×: a) Z-AO and b) Z-AO-GX and film cross-section at 1000×: c) Z-AO and d) Z-AO-GX
The cross-section of Z-OA and Z-OA-XG films (Figs. 1c and d) showed the presence of porosity or orifices caused by ethanol evaporation (30). Apparently, Z-OA- -XG blend films were more porous as a consequence of the higher rigidity determined during the mechanical tests.
Conclusions
Performance of blend films based on zein-oleic acid (Z-OA) and zein-oleic acid and xanthan gum (Z-OA-XG) were evaluated and compared. Z-OA and Z-OA-XG had homogeneous surfaces with good integration of film components. Z-OA exhibited lower water solubility whereas Z-OA-XG showed greater opacity. Hydrocolloid addition had no effect on gas barrier properties since both blend films, Z-OA and Z-OA-XG, showed similar water vapour and oxygen permeability; nonetheless, xanthan gum structure influenced mechanical properties of the film, increasing the strength (higher tensile strength) and rigidity (higher Young’s modulus and lower elasticity). Hence, polysaccharides such as xanthan gum should be added to zein-oleic acid blend films, mainly when the food products tend to be oxidized as a consequence of the influence of light.
Acknowledgements
Authors greatly acknowledge the skillful technical assistance from Glenda Gonçalves (Microscopy Lab., UNESP-FCT, Presidente Prudente, SP, Brazil) during the structural analysis. This study was supported by a grant from FAPESP, No. 2011/08107-3.
References
- 1.Dainelli D, Gontard N, Spyropoulos D, Zondervan-van den Beuken E, Tobback P. Active and intelligent food packaging: legal aspects and safety concerns. Trends Food Sci Technol. 2008;19:103–12. 10.1016/j.tifs.2008.09.011 [DOI] [Google Scholar]
- 2.Restuccia D, Spizzirri G, Parisi O, Cirillo G, Curcio M, Iemma F, et al. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Contr. 2010;21:1425–35. 10.1016/j.foodcont.2010.04.028 [DOI] [Google Scholar]
- 3.Souza AC, Benze R, Ferrăo ES, Ditchfield C, Coelho ACV, Tadini CC. Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT –. Food Sci Technol (Campinas). 2012;46:110–7. 10.1016/j.lwt.2011.10.018 [DOI] [Google Scholar]
- 4.Lagaron JM, Lopez-Rubio A. Nanotechnologies for bioplastics: opportunities, challenges and strategies. Trends Food Sci Technol. 2011;22:611–7. 10.1016/j.tifs.2011.01.007 [DOI] [Google Scholar]
- 5.Sun Q, Sun C, Xiong L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr Polym. 2013;98:630–7. 10.1016/j.carbpol.2013.06.040 [DOI] [PubMed] [Google Scholar]
- 6.Khan A, Khan RA, Salmieri S, Tien CL, Riedl B, Bouchard J, et al. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr Polym. 2012;90:1601–8. 10.1016/j.carbpol.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 7.Fajardo P, Martins JT, Fucińos C, Pastrana L, Teixeira JA, Vicente AA. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J Food Eng. 2010;101:349–56. 10.1016/j.jfoodeng.2010.06.029 [DOI] [Google Scholar]
- 8.del Nobile MA, Conte A, Incoronato AL, Panza O. Antimicrobial efficacy and release kinetics of thymol from zein films. J Food Eng. 2008;89:57–63. 10.1016/j.jfoodeng.2008.04.004 [DOI] [Google Scholar]
- 9.Wu Y, Luo Y, Wang Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid-liquid dispersion method. LWT –. Food Sci Technol (Campinas). 2012;48:283–90. 10.1016/j.lwt.2012.03.027 [DOI] [Google Scholar]
- 10.Zhang B, Luo Y, Wang Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem. 2011;124:210–20. 10.1016/j.foodchem.2010.06.019 [DOI] [Google Scholar]
- 11.Tharanathan R. Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol. 2003;14:71–8. 10.1016/S0924-2244(02)00280-7 [DOI] [Google Scholar]
- 12.Arcan I, Yemenicioğlu A. Development of flexible zein–wax composite and zein–fatty acid blend films for controlled release of lysozyme. Food Res Int. 2013;51:208–16. 10.1016/j.foodres.2012.12.011 [DOI] [Google Scholar]
- 13.Lawton JW. Plasticizers for zein: their effect on tensile properties and water absorption of zein films. Cereal Chem. 2004;81:1–5. 10.1094/CCHEM.2004.81.1.1 [DOI] [Google Scholar]
- 14.Pérez M, Montero P, Gómez MC. Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends. Food Hydrocoll. 2009;23:53–61. 10.1016/j.foodhyd.2007.11.011 [DOI] [Google Scholar]
- 15.Cuq B, Gontard N, Guilbert S. Edible films and coatings as active layers. In: M.L. Rooney, editor. Active food packaging. Glasgow, UK: Springer Science+Business Media Dordrecht; 1995. pp. 111–35. [Google Scholar]
- 16.Wang Y, Padua GW. Water barrier properties of zein-oleic acid films. Cereal Chem. 2006;83:331–4. 10.1094/CC-83-0331 [DOI] [Google Scholar]
- 17.Barbosa De Almeida C, Tafari Catelam K, Lopes Cornélio M, Lopes Filho J. Morphological and structural characteristics of zein biofilms with added xanthan gum. Food Technol Biotechnol. 2010;48:19–27. [Google Scholar]
- 18.ASTM D618-13. Standard practice for conditioning plastics for testing. ASTM International, West Conshohocken, PA, USA; 2013. http://dx.doi.org/ 10.1520/D0618 [DOI]
- 19.Cao N, Fu Y, He J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007;21:1153–62. 10.1016/j.foodhyd.2006.09.001 [DOI] [Google Scholar]
- 20.Cerqueira MA, Lima AMP, Vicente AA, Teixeira JA, Moreira RA. Novel functional polysaccharides as edible coatings for cheese. Proceedings of the 3rd CIGR Section VI International Symposium on Food and Agricultural Products: Processing and Innovations, Naples, Italy; 2007. pp. 1–17. http://hdl.handle.net/1822/7466
- 21.ASTM E96/E96M-14. Standard test method for water vapor transmission of materials. West Conshohocken, PA, USA: ASTM International; 2014. http://dx.doi.org/ 10.1520/E0096_E0096M [DOI]
- 22.ASTM F1927-14. Standard test method for determination of oxygen gas transmission rate, permeability and permeance at controlled relative humidity through barrier materials using a coulometric detector. West Conshohocken, PA, USA: ASTM International; 2014. http://dx.doi.org/ 10.1520/F1927 [DOI]
- 23.ASTM D882-12. Standard test method for tensile properties of thin plastic sheeting. West Conshohocken, PA, USA: ASTM International; 2012. http://dx.doi.org/ 10.1520/D0882 [DOI]
- 24.ASTM D6287-09. Standard practice for cutting film and sheeting test specimens. West Conshohocken, PA, USA: ASTM International; 2009. http://dx.doi.org/ 10.1520/D6287-09 [DOI]
- 25.Bourbon A, Pinheiro A, Cerqueira MA, Rocha CMR, Avides MC, Quintas MAC, et al. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J Food Eng. 2011;106:111–8. 10.1016/j.jfoodeng.2011.03.024 [DOI] [Google Scholar]
- 26.Denavi GA, Pérez M, Ańón MC, Montero P, Mauri AN, Gómez MC. Structural and functional properties of soy protein isolate and cod gelatin blend films. Food Hydrocoll. 2009;23:2094–101. 10.1016/j.foodhyd.2009.03.007 [DOI] [Google Scholar]
- 27.Pena-Serna C, Lopes-Filho JF. Influence of ethanol and glycerol concentration over functional and structural properties of zein-oleic acid films. Mater Chem Phys. 2013;142:580–5. 10.1016/j.matchemphys.2013.07.056 [DOI] [Google Scholar]
- 28.Nieto M. Structure and function of polysaccharide gum-based edible films and coatings. In: Embuscado ME, Huber KC, editors. Edible films and coatings for food applications structure and function of polysaccharide gum-based edible films and coatings. New York, NY, USA: Springer; 2009. pp. 57–112. [Google Scholar]
- 29.Jia D, Fang Y, Yao K. Water vapor barrier and mechanical properties of konjac glucomannan–chitosan–soy protein isolate edible films. Food Bioprod Process. 2009;87:7–10. 10.1016/j.fbp.2008.06.002 [DOI] [Google Scholar]
- 30.Sothornvit R, Rhim JW, Hong SI. Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J Food Eng. 2009;91:468–73. 10.1016/j.jfoodeng.2008.09.026 [DOI] [Google Scholar]
- 31.Xingfeng G, Yanan L, Heping C, Hongchao B, Yuxiang M. Factors affecting the physical properties of edible composite film prepared from zein and wheat gluten. Molecules. 2012;17:3794–804. 10.3390/molecules17043794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matta M, Sarantópoulos C, Zocchi S. Barrier properties and solubility of pea starch films associated with xanthan gum and glycerol. Polímeros. 2011;21:67–72. [Google Scholar]
- 33.Krochta J. Proteins as raw materials for films and coatings: definitions, current status and opportunities. In: Gennadios A, editor. Protein-based films and coatings. Boca Raton, FL, USA: CRC Press; 2002. pp. 1–41. [Google Scholar]
- 34.Miller KS, Krotcha JM. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci Technol. 1997;8:228–37. 10.1016/S0924-2244(97)01051-0 [DOI] [Google Scholar]
- 35.Ku K, Song KB. Physical properties of nisin-incorporated gelatin and corn zein films and antimicrobial activity against Listeria monocytogenes. J Microbiol Biotechnol. 2007;17:520–3. [PubMed] [Google Scholar]
- 36.Hewage S, Vithanarachchi SM. Preparation and characterization of biodegradable polymer films from cowpea (Vigna unguiculata) protein isolate. J Natn Sci Foundation Sri Lanka. 2009;37:53–9. 10.4038/jnsfsr.v37i1.457 [DOI] [Google Scholar]