Abstract
The fatigue spreads among the people who live under stressful life and brings about a negative impact on physical function. Here we evaluated the anti-fatigue effects of fermented porcine placenta (FPP) and main constituents, lysine (Lys) and leucine (Leu) with treadmill stress test and forced swimming test (FST) in animal models. The mice were administrated with FPP, Lys, and Leu for 21 days. After treadmill exercise, FPP, Lys, and Leu significantly reduced fatigue-related biochemical parameters, including lactate, lactate dehydrogenase, glucose, creatine kinase, urea nitrogen, cortisol, and pro-inflammatory cytokines, whereas superoxide dismutase activity and glycogen levels were significantly increased by FPP, Lys, and Leu. In the FST, FPP, Lys, and Leu significantly decreased immobility times and up-regulated brain-derived neurotrophic factor expression in brain. Furthermore, FPP, Lys, and Leu significantly decreased production of tumor necrosis factor-α, interleukin (IL)-6, IL-1β, and IL-4 through blockade of caspase-1/nuclear factor-κB pathway in stimulated splenocytes. In addition, FPP, Lys, and Leu significantly promoted proliferation of splenocytes. In conclusion, these findings suggest the potential of FPP as a novel functional food for the regulation of fatigue.
Keywords: Fermented porcine placenta, lysine, leucine, fatigue, biochemical parameters, treadmill test
Introduction
Fatigue is one of the most common and disabling non-motor problems and brings about a negative impact on physical function.1 Fatigue spreads among the people who live under stressful life and can be seriously aggravated by the excessive stress.1 In addition, dysfunction of the immune, endocrine, or antioxidant systems can lead to fatigue or fatigue-related disorders.2 Numerous biochemical parameters, including lactate, glycogen, and blood urea nitrogen (BUN) are involved in fatigue.3 Lactate dehydrogenase (LDH) and creatine kinase (CK) are precise indicators of muscle damage.4 Fatigue is also associated with immune abnormalities in oxidative stress pathways or multiple inflammatory pathways.5 Oxidative stress resulting from excessive exercise induces the production of excess free radicals that contribute intensively to fatigue.6 Nitric oxide (NO) metabolites showed a much higher increase in chronic fatigue syndrome (CFS) patients.7 Intracellular defense systems which consist of antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT) can eliminate free radicals.5 In addition, the symptoms of fatigue are consistent with inflammatory cytokine dysregulation.8
Seriously, pharmacological drugs for treating fatigue cannot yet satisfy the need of the people. In our earlier study, porcine placenta extract regulated malnutrition-induced fatigue by decreasing fatigue-related factors.9 Also, it was reported that the anti-inflammatory property of human placenta extract ameliorated chronic inflammation in CFS.10 Scientific evidences demonstrated the numerous health benefits of placenta extract including immune modulation, depression, menopause, and cellular regeneration.11–13 Fermentation is known to change protein content and enhances health benefits.14 Also, fermentation can be considered as an effective process for increasing anti-fatigue effects.15 Mitsui et al.16 reported that fermented porcine placenta (FPP) is safe and nontoxic. Based on previous reports, we hypothesized that FPP and its constituents, lysine (Lys) and leucine (Leu) increased by fermentation could be preferentially enhancing anti-fatigue effect (Table 1). Thus, we investigated the anti-fatigue effects of FPP, Lys, and Leu on exercise-induced fatigue mice models, treadmill stress test and forced swimming test (FST) which are the most useful screening tests for anti-fatigue drugs.17 And we examined the effect and regulatory mechanism of FPP, Lys, and Leu on production of inflammatory cytokines in the stimulated splenocytes.
Table 1.
Ingredient variations of porcine placenta by the fermentation process
| (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lys | Leu | Arg | Ala | Pro | Thr | His | Iso | Tyr | Met | Phe | Try | Val | |
| PP | 1.79 | 2.45 | 1.67 | 1.45 | 0.61 | 0.95 | 0.99 | 0.94 | 0.93 | 0.48 | 1.31 | 0.38 | 1.40 |
| FPP | 2.12 | 3.02 | 1.94 | 1.46 | 0.70 | 1.02 | 1.16 | 0.99 | 1.18 | 0.64 | 1.63 | 0.48 | 1.62 |
PP: porcine placenta, FPP: fermented porcine placenta, Lys: lysine, Leu: leucine, Arg: arginine, Ala: alanine, Pro: proline, Thr: threonine, His: histidine, Iso: isoleucine, Tyr: tyrosine, Met: methionine, Phe: phenylalanine, Try: tryptophane, Val: valine.
Materials and methods
Animals
The male ICR (4 weeks old) was obtained from the Dae-Han Experimental Animal Center (Eumsung, Chungbuk, Republic of Korea). Mice were acclimated to individual cages during seven days in a room with controlled lighting (12 h light–dark cycle), humidity (50–60%), and temperature (20–23℃). Experimental procedures and animal care were approved by the Animal Care Committee of Kyung Hee University (KHUASP [SE]-15-080).
Preparation of FPP
Considering safety, stability, and effective utilization of standardized materials, we purchased the commercial FPP (Fermented Placenta Extract Powder A (K), HORUS Co., Ltd. Japan) which is currently used in Japan and Korea for clarifying the role of FPP on anti-fatigue activity. Fermentation conditions are as follows. Porcine placenta was derived from pathogen-free porcine placenta, which was partially hydrolyzed by protease. Then, yeast and black strap powder were added to initiate fermentation, which was later filtered through 2 µm filter and spray dried. FPP was dissolved in distilled water (DW) and doses of 0.1, 1, and 10 mg/kg were determined according to a previous report.10
Amino acids analysis
Amino acids were evaluated by using automatic amino acid analyzer (Sykam GmbH, Germany). FPP is made up of Leu (3.02%), Lys (2.12%), arginine (1.94%), valine (1.62%), phenylalanine (1.63%), alanine (1.46%), histidine (1.16%), threonine (1.02%), proline (0.70%), and so on. We investigated the anti-fatigue effect of FPP and its main constituents, Lys and Leu. Lys and Leu were purchased from Sigma Chemical Co. (St. Louis, MO). The Lys and Leu were dissolved in DW and doses of 10 mg/kg were determined according to previous reports.18,19
Treadmill stress test
The mice in the each group were forced to run on the treadmill for 30 min once a week during three weeks. The pattern of loaded exercise composed of forced running at a 10 m/min during the 10 min, in sequence 16 m/min during the 10 min, last 21 m/min during the 10 min. On the 21th day, the speeds used for determination of the exhaustion were 10 m/min for 5 min, in sequence for 3 min each, then 40 m/min during the 30 min.20 The administration of FPP (0.1, 1, and 10 mg/kg), Lys (10 mg/kg), and Leu (10 mg/kg) was continued for 21 days at the same time. Administration of DW was continued for 21 days at the same time in control group.
Forced swimming test
Based on the first measurement of immobility times, the mice were separated as control, FPP (0.1, 1, and 10 mg/kg), Lys (10 mg/kg), and Leu (10 mg/kg) groups. FPP (0.1, 1, and 10 mg/kg), Lys (10 mg/kg), and Leu (10 mg/kg) groups were orally administered to mice once per day for 21 days. Administration of DW was continued for 21 days at the same time in control group. The FST proceed at the end of administration period for the 21 days. The FST was performed as described previously.21
Fatigue-related biochemical parameters analysis in serum, muscle, or liver
After blood samples were collected from the heart in mice, the serum was separated. Muscle and liver were collected rapidly under standard conditions. The levels of lactate (#ab65331), LDH (#ab102526), glucose (#ab65333), CK (#ab155901), SOD (#ab65354), glycogen (#ab65620), and catalase (#ab83464) were determined using commercially available kits (Abcam, Cambridge, UK). The level of BUN (#K024-H1) was determined using commercially available kit (Arbor Assays, Ann Arbor, MI). The level of cortisol (#SE120082) was determined using commercially available kit (Sigma Chemical Co).
L6 cell culture
The rat skeletal muscle cell line (L6) was purchased from Korean Cell Line Bank and was cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY) with 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 µg/mL) at 37℃ in 5% CO2 with 95% atmosphere.
3 -(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
L6 cells (1 × 104) or splenocytes cells (2 × 105) treated with FPP (0.1, 1, and 10 µg/mL), Lys (1 and 10 µg/mL), and Leu (1 and 10 µg/mL) for 24 h were incubated with MTT solution (5 mg/mL, Sigma Chemical Co.) for 4 h at 37℃ in a 5% CO2 with 95% atmosphere. Dimethyl sulfoxide was then added to the formazan and absorbance was measured at 540 nm using an ELISA reader.
Enzyme-linked immunosorbent assay (ELISA)
The levels of cytokines in serum, spleen, and splenocytes were measured by ELISA, as previously performed.22 The levels of cytokines in spleen were divided according to the total protein levels which were measured using a bicinchoninic acid (BCA) protein assay kit (Sigma Chemical Co.).
Measurement of NO concentration
NO concentration in serum were measured by the Griess method.22
Isolation of splenocytes
The spleen was teased into a single cell suspension in RPMI-1640 with 10% FBS, penicillin (100 U/mL), streptomycin (100 µg/mL), and 2 mmol/L l-glutamine. The splenocytes cells (2 × 105) were treated with FPP (0.1, 1, and 10 µg/mL), Lys (1 and 10 µg/mL), and Leu (1 and 10 µg/mL) for each times.
Western blot analysis
Western blot was fulfilled on the extract of brain tissue or splenocytes, as previously performed.20
Nuclear protein extraction
Harvested splenocytes cells (3 × 105) were resuspended in 400 µL of ice-cold hypotonic buffer (0.1 mmol/L ethylenediaminetetraacetic acid (EDTA), 2 mmol/L MgCl2, 10 mmol/L Hepes/KOH, 0.5 mmol/L phenylmethane sulfonyl fluoride (PMSF), 1 mmol/L dithiothreitol (DTT), and 10 mmol/L KCl, pH 7.9). After placing it on the ice for 10 min, it was vortexed and centrifuged at 15,000 g for 30 s. Supernatant aliquots including the cytoplasmic proteins were stored at −70℃. Pelleted nuclei were gently resuspended in 50 µL of ice-cold saline buffer (0.1 mmol/L EDTA, 50 mmol/L Hepes/KOH, 0.5 mmol/L PMSF, 1 mmol/L DTT, 50 mmol/L KCl, 300 mmol/L NaCl, and 10% glycerol, pH 7.9). After placing it on ice for 20 min, it was vortexed and centrifuged at 15,000 g for 5 min at 4℃. Supernatant aliquots including the nuclear proteins were stored at −70℃. Each protein level was estimated using a BCA protein assay method (Sigma, St. Louis, MO).
Assessment of proliferation
The proliferation of splenocytes was measured using a colorimetric immunoassay based on the bromodeoxyuridine (BrdU) incorporated measurement through the DNA synthesis (#11 647 229 001, Roche Diagnostics GmbH, Mannheim, Germany).
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from splenocytes using an easy-BLUE™ RNA extraction kit (iNtRON Biotech, Sungnam, Korea) and total RNA concentration was determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). We performed the quantitative real-time PCR using an SYBR Green master mix, and mRNA was detected using an ABI StepOne real time PCR System (Applied Biosystems, Foster City, CA). We used the following primers: mouse KI-67 (5′ CTT CAC TCT TAC TTT CCA CA 3′; 5′AAC ACC TAC AAA ATG ACT TC 3′) and mouse GAPDH (5′ TCG ACA GTC AGC CGC ATC TTC TTT 3′; 5′ ACC AAA TCC GTT GAC TCC GAC CTT 3′). Typical profile times used were as follows: initial step 95℃ for 10 min followed by a second step at 95℃ for 15 s and 60℃ for 30 s for 40 cycles. Levels of target mRNAs were normalized versus GAPDH. Data were analyzed using the ΔΔCT method.
Statistical analysis
All data were checked for normality using the Shapiro-Wilk test. Treatment effects were analyzed using one-way analysis of variance (ANOVA) and the independent t-test. In vitro data are shown as mean ± standard error mean (SEM) from at least three independent experiments performed in duplicates or triplicates. In vivo data are represented as the mean ± SEM (n = 5/group). Statistical significance was accepted for P values < 0.05.
Results
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in serum after treadmill stress test
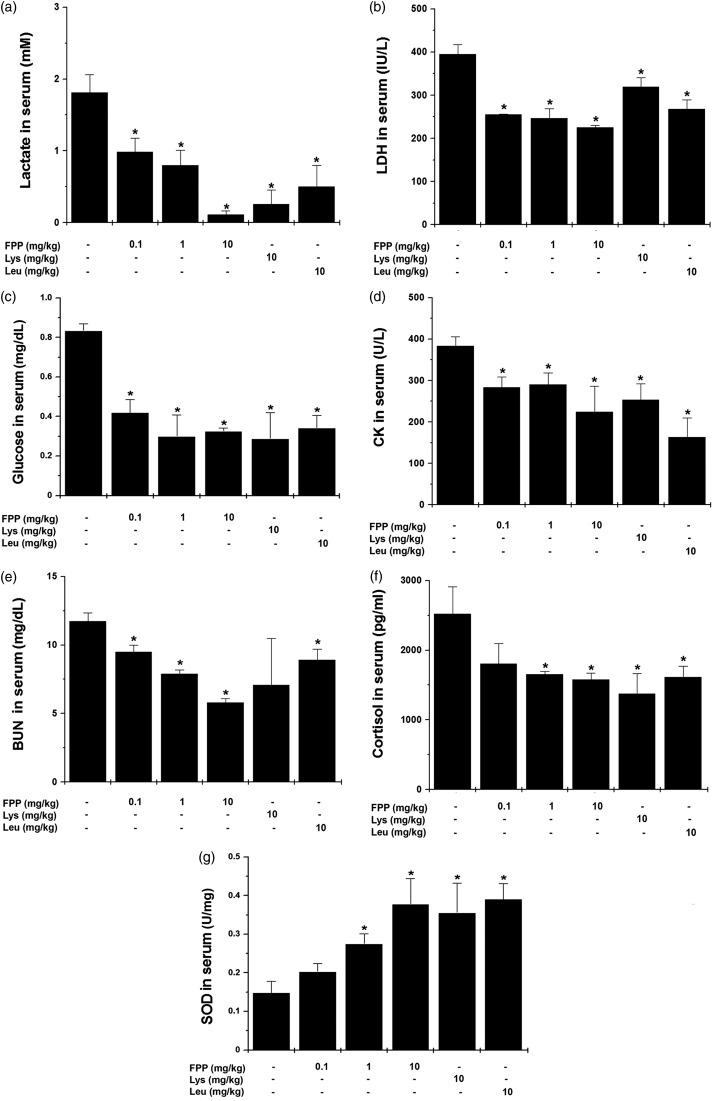
First, we investigated whether FPP, Lys, and Leu would have an anti-fatigue effect after treadmill exercise by measuring blood biochemical parameters. As shown in Figure 1, administration of FPP and Leu down-regulated serum lactate, LDH, glucose, CK, BUN, and cortisol levels compared with the control group (Figure 1a to f, P < 0.05). Lys decreased serum lactate, LDH, glucose, CK, and cortisol levels after treadmill exercise (Figure 1a to f, P < 0.05). In addition, administration of FPP, Lys, and Leu up-regulated SOD levels in the serum compared with the control group (Figure 1g, P < 0.05).
Figure 1.
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in serum after treadmill stress test. After the last treadmill exercise, serum samples were obtained from heart. (a) Lactate, (b) LDH, (c) glucose, (d) CK, (e) BUN, (f) cortisol, and (g) SOD were measured with each kit. Values are the mean ± SEM. n = 5 per group. BUN: blood urea nitrogen, CK: creatine kinase, FPP: fermented porcine placenta, LDH: lactate dehydrogenase, Lys: lysine, Leu: leucine, SEM: standard error mean, SOD: superoxide dismutase. *P < 0.05, significantly different from the control mice
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in muscle after treadmill stress test
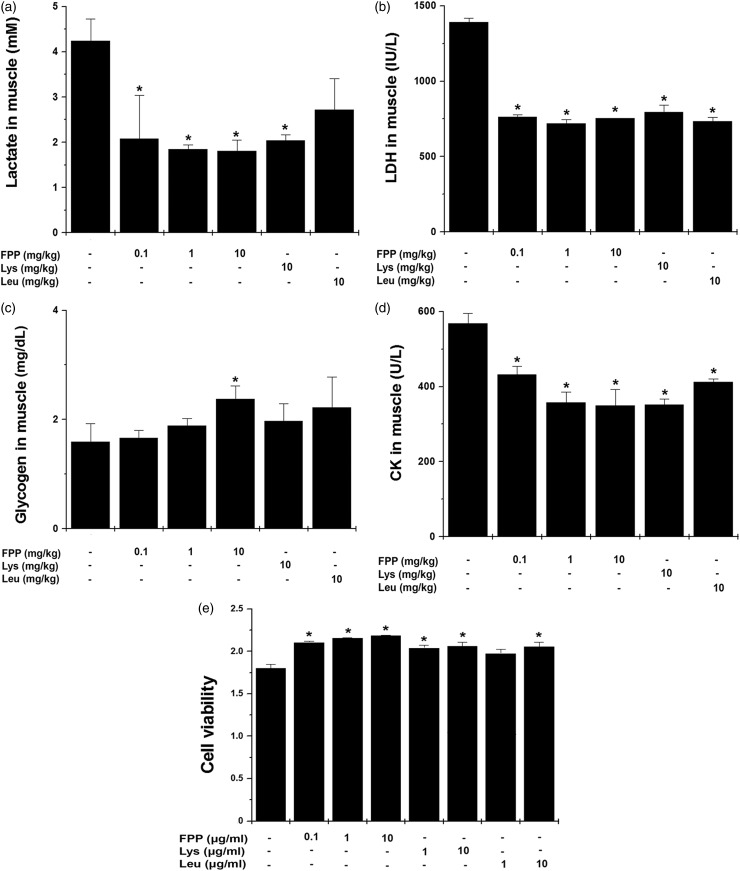
Next, we measured fatigue-related biochemical parameters in muscle of FPP, Lys, and Leu-treated mice. As shown in Figure 2, administration of FPP significantly down-regulated lactate, LDH, and CK levels and administration of Lys and Leu significantly down-regulated LDH and CK in muscle compared with the control group (Figure 2a, b, and d, P < 0.05). By contrast, muscle glycogen content of FPP group was significantly higher than that of the control group (Figure 2c, P < .05). Further, we examined whether FPP, Lys, and Leu would regulate muscle damage in vitro model using L6 cells. FPP, Lys, and Leu significantly improved viability of L6 cells (Figure 2e, P < 0.05).
Figure 2.
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in muscle after treadmill stress test. After the last treadmill exercise, muscle samples were obtained from each mouse. (a) Lactate, (b) LDH, (c) glycogen, and (d) CK were measured with each kit. (e) L6 cells were treated with FPP, Lys, and Leu for 24 h except FBS. The viability of L6 cells was measured by a MTT assay. Values are the mean ± SEM. n = 5 per group. CK: creatine kinase, FBS: fetal bovine serum, FPP: fermented porcine placenta, LDH: lactate dehydrogenase, Lys: lysine, Leu: leucine, MTT: 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, SEM: standard error mean. *P < 0.05, significantly different from the control mice
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in liver after treadmill stress test
Liver is an important organ involved in long-term exercise. The prolonged exercise increases oxidative stress in the liver.23 Administration of FPP, Lys, and Leu significantly up-regulated SOD and CAT levels in liver compared with the control group (Table 2, P < 0.05). Glycogen was also significantly up-regulated by FPP, Lys, and Leu compared with the control group (Table 2, P < 0.05).
Table 2.
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters in liver and spleen after treadmill
| Control | FPP (0.1 mg/kg) | FPP (1 mg/kg) | FPP (10 mg/kg) | Lys (10 mg/kg) | Leu (10 mg/kg) | ||
|---|---|---|---|---|---|---|---|
| Liver | Glycogen | 2.17 ± 0.15 | 4.51 ± 0.74* | 5.07 ± 0.21* | 8.24 ± 1.45* | 3.37 ± 0.40* | 4.99 ± 1.05* |
| SOD | 3.36 ± 0.41 | 8.09 ± 1.56* | 10.71 ± 1.22* | 12.76 ± 1.22* | 4.43 ± 0.36* | 8.08 ± 2.44* | |
| Catalase | 1.30 ± 0.11 | 2.48 ± 0.58* | 2.66 ± 0.41* | 4.12 ± 0.90* | 1.70 ± 0.25* | 2.52 ± 0.55* | |
| Spleen | TNF-α | 0.34 ± 0.05 | 0.14 ± 0.09* | 0.14 ± 0.09* | 0.06 ± 0.13* | 0.18 ± 0.06* | 0.15 ± 0.06* |
| IL-6 | 0.08 ± 0.01 | 0.02 ± 0.01* | 0.02 ± 0.01* | 0.01 ± 0.001* | 0.007 ± 0.003* | 0.004 ± 0.002* | |
| IL-1β | 6.53 ± 0.79 | 5.08 ± 1.66* | 3.63 ± 0.97* | 1.06 ± 0.16* | 4.58 ± 0.96* | 3.24 ± 0.60* | |
| IL-4 | 0.15 ± 0.09 | 0.05 ± 0.001* | 0.01 ± 0.01* | 0.01 ± 0.01* | 0.01 ± 0.01* | 0.01 ± 0.01* | |
| IFN-γ | 1.76 ± 0.92 | 3.57 ± 1.75 | 4.27 ± 0.99 | 8.90 ± 2.16* | 2.98 ± 0.57 | 1.94 ± 2.73 | |
The administration of FPP, Lys, and Leu was continued for 21 days at the same time. After the last treadmill test, liver and spleen samples were obtained the from each mouse. Glycogen (mg/dL), SOD (U/mg), and catalase (mU/mL) were measured by the each assay kit. The levels of TNF-α, IL-6, IL-1β, IL-4, and IFN-γ were divided according to total protein levels and determined by the ELISA method. Values are the mean ± SEM. n = 5 per group. FPP: fermented porcine placenta, IFN: interferon, IL: interleukin, Lys: lysine, Leu: leucine, SOD: superoxide dismutase, TNF: tumor necrosis factor.
P < 0.05, significantly different from the control mice.
Effect of FPP, Lys, and Leu on fatigue-related cytokines in serum and spleen after treadmill stress test
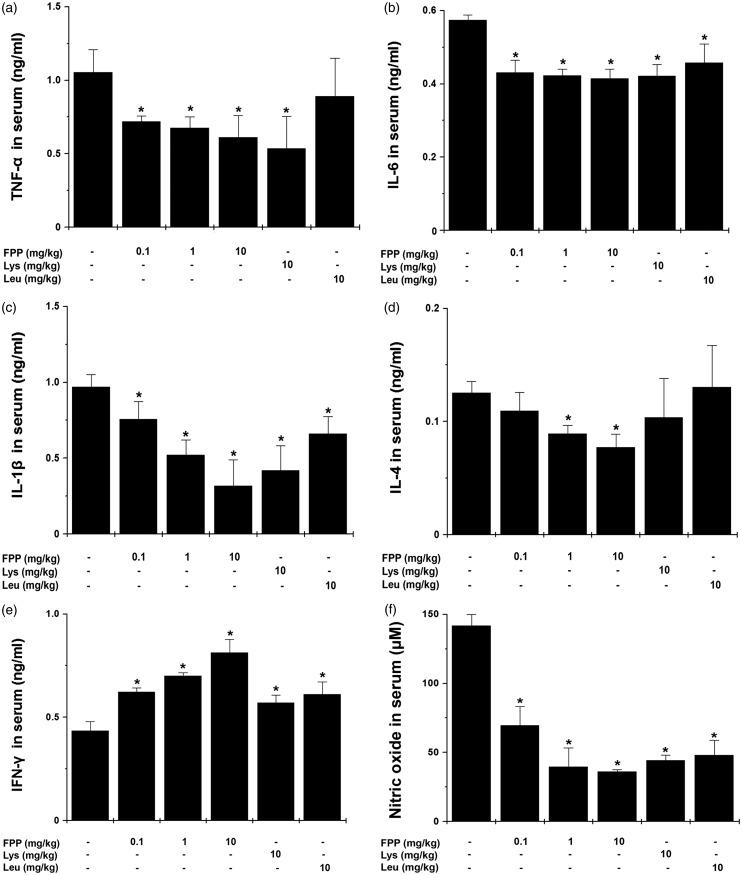
We studied whether FPP, Lys, and Leu would regulate the levels of inflammatory cytokines in serum after treadmill exercise. Administration of FPP significantly down-regulated levels of tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), IL-1β, IL-4, and NO in serum compared with the control group (Figure 3a to d and f, P < 0.05,). Also, FPP significantly up-regulated serum interferon (IFN)-γ level compared with the control group (Figure 3e, P < 0.05). Lys significantly down-regulated levels of serum TNF-α, IL-6, IL-1β, and NO, whereas it up-regulated serum IFN-γ level compared with the control group (Figure 3a to f, P < 0.05). Leu also significantly decreased levels of serum IL-6, IL-1β, and NO, whereas it up-regulated serum IFN-γ level compared with the control group (Figure 3b to f, P < 0.05). In spleen, administration of FPP, Lys, and Leu significantly down-regulated the levels of inflammatory cytokines, including TNF-α, IL-6, IL-1β, and IL-4 compared with the control group (Table 2, P < 0.05). FPP significantly up-regulated IFN-γ levels in spleen compared with the control group (Table 2, P < 0.05).
Figure 3.
Effect of FPP, Lys, and Leu on fatigue-related cytokines in serum after treadmill stress test. After the last treadmill exercise, serum sample were obtained from heart. The levels of (a) TNF-α, (b) IL-6, (c) IL-1β, (d) IL-4, and (e) IFN-γ were analyzed by the ELISA method. (f) The levels of nitric oxide were measured by the Griess method. Values are the mean ± SEM. n = 5 per group. ELISA: enzyme-linked immunosorbent assay, FPP: fermented porcine placenta, IFN: interferon, IL: interleukin, Lys: lysine, Leu: leucine, SEM, standard error mean, TNF: tumor necrosis factor. *P < 0.05, significantly different from the control mice
Effect of FPP, Lys, and Leu on FST
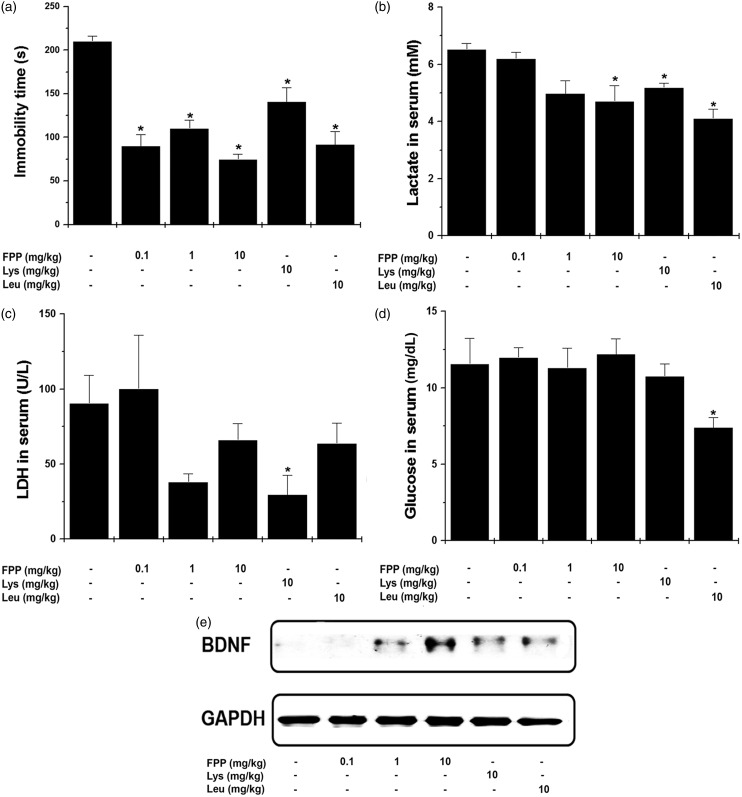
Furthermore, we investigated an anti-fatigue effect of FPP, Lys, and Leu by performing another fatigue model, FST. The immobility times of the mice administrated with FPP, Lys, and Leu were significantly down-regulated compared with the control group at 21 days (Figure 4a, P < 0.05). Figure 4 shows that FPP, Lys, and Leu significantly down-regulated serum lactate level compared with the control group (Figure 4b, P < 0.05). Lys significantly decreased serum LDH level compared with the control group (Figure 4c, P < 0.05). Leu significantly reduced glucose level compared with the control group (Figure 4d, P < 0.05). However, FPP did not significantly decrease the levels of LDH and glucose. In brain, brain-derived neurotrophic factor (BDNF) expression was also up-regulated by FPP, Lys, and Leu (Figure 4e).
Figure 4.
Effect of FPP, Lys, and Leu on fatigue-related biochemical parameters after FST. (a) The immobility times of the mice were measured at the 21st days. (b–d) After the FST, serum samples were obtained from heart. The serum lactate and LDH levels were measured with each kit. (e) The protein levels of BDNF in brain were analyzed by the Western blotting. Values are the mean ± SEM. n = 5 per group. BDNF: brain-derived neurotrophic factor; FPP: fermented porcine placenta, FST: forced swimming test, LDH: lactate dehydrogenase, Lys: lysine, Leu: leucine; SEM: standard error mean. *P < 0.05, significantly different from the control mice
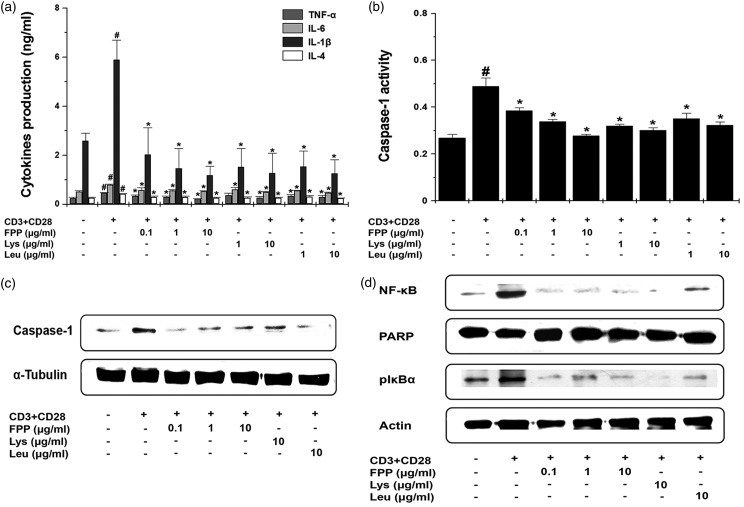
Effect of FPP, Lys, and Leu on fatigue-related cytokines in splenocytes
Given up-regulation of inflammatory cytokines levels in spleen after treadmill exercising, we investigated the mechanism of an anti-fatigue effect of FPP, Lys, and Leu in anti-CD3 and anti-CD28 antibodies-stimulated splenocytes. Treatment with FPP, Lys, and Leu significantly down-regulated the production of TNF-α, IL-6, IL-1β, and IL-4 from anti-CD3 and anti-CD28 antibodies-stimulated splenocytes (Figure 5a, P < 0.05,). It is reported that the caspase-1 regulates the production of TNF-α, IL-6, and IL-1β.24 Thus, we examined the regulatory effects of FPP, Lys, and Leu on the caspase-1 activity. FPP, Lys, and Leu significantly decreased caspase-1 activity in activated splenocytes (Figure 5b, P < 0.05). Also, FPP, Lys, and Leu significantly decreased caspase-1 protein levels in activated splenocytes (Figure 5c, P < 0.05). Nuclear factor (NF)-κB is a crucial transcription factor required for the pro-inflammatory cytokines expression, such as TNF-α, IL-6, and IL-1β.25 Thus, we performed the regulatory effects of FPP, Lys, and Leu on the translocation into nucleus of NF-κB and phosphorylation of IκBα. As shown in Figure 5(d), FPP, Lys, and Leu inhibited level of NF-κB in nucleus and phosphorylation of IκBα in cytosol of activated splenocytes (P < 0.05).
Figure 5.
Effect of FPP, Lys, and Leu on fatigue-related cytokines in splenocytes. (a) Splenocytes (2 × 105) were stimulated with immobilized anti-CD3 and soluble anti-CD28 antibodies and treated with FPP, Lys, and Leu for 24 h. The production of TNF-α, IL-6, IL-1β, and IL-4 was analyzed by the ELISA method. (b) Splenocytes (2 × 105) were stimulated and treated with FPP, Lys, and Leu for 24 h. Caspase-1 activity was measured by a caspase-1 assay kit. Results show the mean ± SEM of data from three separate experiments with triplicate samples. (c) Splenocytes (2 × 105) were stimulated and treated with FPP, Lys, and Leu for 45 min. Caspase-1 protein levels were analyzed by the Western blotting. (d) Splenocytes (2 × 105) were stimulated and treated with FPP, Lys, and Leu for 45 min. The level of NF-κB in nuclear extract and level of phosphorylated IκBα in cytoplasmic extract were analyzed by the Western blotting. ELISA: enzyme-linked immunosorbent assay, FPP: fermented porcine placenta, IL: interleukin, Lys: lysine, Leu: leucine, NF: nuclear factor, pIκBα: phosphorylation of IκBα, SEM: standard error mean, TNF: tumor necrosis factor. #P < 0.05, significantly different from the un-stimulated splenocytes. *P < 0.05, significantly different from the stimulated splenocytes
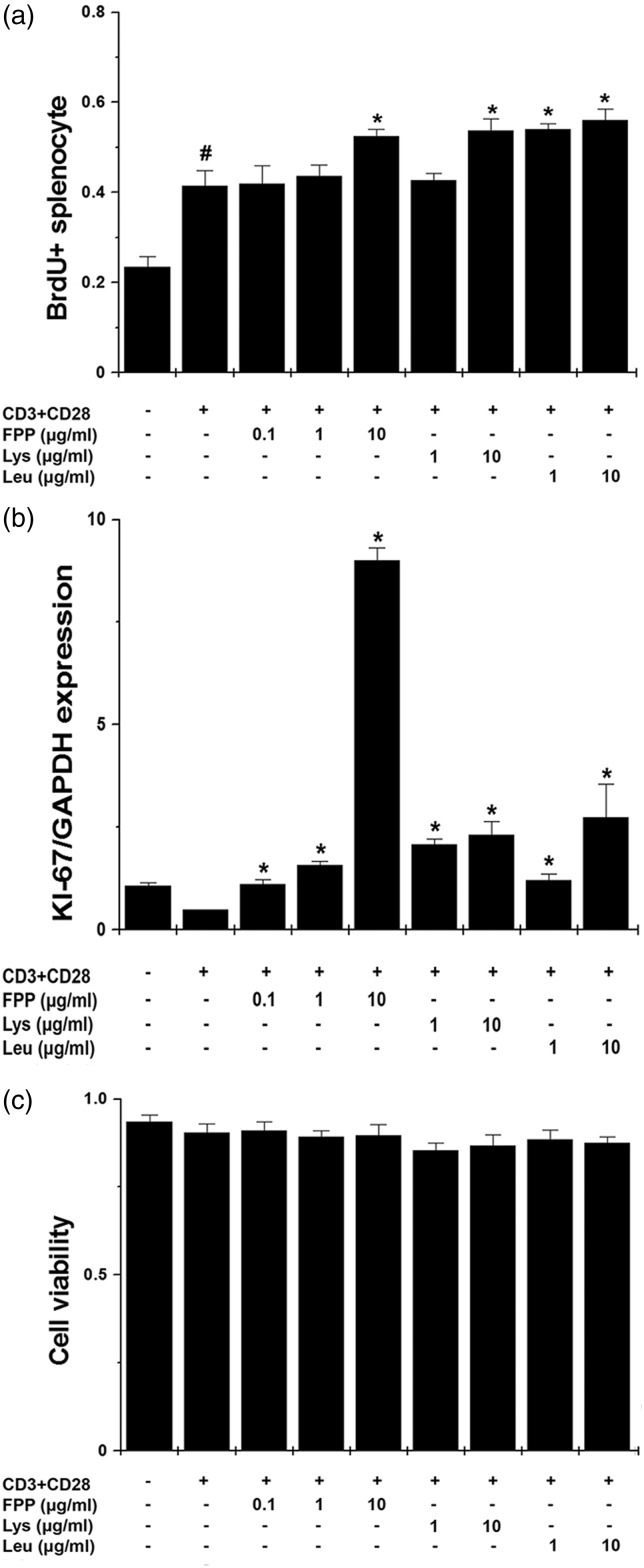
Effect of FPP, Lys, and Leu on proliferation of splenocytes
Finally, we evaluated proliferation markers, such as BrdU incorporation and KI-67 mRNA expression to investigate the effects on the immunity enhancement of FPP, Lys, and Leu in vitro. Treatment with FPP, Lys, and Leu significantly promoted BrdU incorporation in anti-CD3 and anti-CD28 antibodies-stimulated splenocytes (Figure 6a, P < 0.05). Also, FPP, Lys, and Leu significantly up-regulated KI-67 mRNA expression in stimulated splenocytes (Figure 6b, P < 0.05). Cytotoxicity was not shown in all instances (Figure 6c).
Figure 6.
Effect of FPP, Lys, and Leu on proliferation of splenocytes. (a) Splenocytes (2 × 105) were stimulated with immobilized anti-CD3 and soluble anti-CD28 antibodies and treated with FPP, Lys, and Leu for 48 h. BrdU incorporation assay was performed. (b) The mRNA levels of KI-67 were measured by the quantitative real-time PCR. (c) The cytotoxicity was measured by a MTT assay. Results show the mean ± SEM of data from three separate experiments with triplicate samples. FPP: fermented porcine placenta, Lys: lysine, Leu: leucine, MTT: 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, PCR, polymerase chain reaction, SEM: standard error mean. #P < 0.05, significantly different from the un-stimulated splenocytes. *P < 0.05, significantly different from the stimulated splenocytes
Discussion
In our research, we observed the anti-fatigue effect of FPP, Lys, and Leu on several biochemical markers in treadmill stress test and FST in vivo models and splenocytes in vitro model.
Exercise causes accumulation and production of metabolic products, such as lactate.26 Serum lactate and LDH are increased in exercise-induced fatigue model.27 An increase in CK with LDH is index of cellular necrosis and tissue damage in skeletal muscles.28 Because most of the CK in the body is generally in the muscle, an increase in CK in blood shows muscle damage.4 A positive correlation is between BUN and exercise tolerance.29 The significant increases in BUN, LDH, and CK were observed in the specimens obtained after the marathon compared with the premarathon specimens.30 Our results show that the levels of lactate, LDH, CK, and BUN in FPP or Leu-treated mice were lower than those in the control group on treadmill stress test and lactate levels in FPP, Lys, and Leu-treated mice were lower than those in the control group on FST. In addition, FPP, Lys, and Leu increased the viability of L6 cells, suggesting that they ameliorate muscle damage. Thus, we suggest that FPP may have anti-fatigue effect through down-regulating the accumulation of metabolites in exercise-induced fatigue.
Cortisol is known as a biomarker of stress from physical or psychological stimuli. Serum cortisol measurement has been widely performed to evaluate physical stress response to strenuous exercise.31 In stressful situations, cortisol can provide glucose to the body and leads to high blood sugar levels.32 Schisandra chinensis reduced levels of serum cortisol and glucose in rats under strenuous swimming exercise and decreased the stress.33 Our result also shows that cortisol and glucose levels increased by treadmill exercise were inhibited by FPP, Lys, and Leu. Thus, we speculate that FPP, Lys, and Leu might reduce extreme exercise-induced stress, indirectly decreasing stress-induced high blood glucose levels. The main repositories of glycogen which is the polymeric storage form of glucose are skeletal muscle and liver.34 Glycogen is a critical source of energy for skeletal muscle contraction in prolonged exercise.35 During excessive exercise, the depletion of glycogen severely restricted energy supply.36 However, protein ingestion alleviated the fatigue caused by the depletion of muscle glycogen during exercise endurance.36 Our results show that administration of FPP after treadmill exercise increased glycogen level in muscle and liver. Thus, we suppose that administration of FPP would protect skeletal muscle and liver from glycogen depletion. And this result would indicate that FPP, Lys, and Leu have an anti-fatigue effect, particularly regulating fatigue-related factors in muscle and liver. Thus, we assume that FPP-treated mice might not need more energy during prolonged exercise than control mice.
The betterment of antioxidant enzyme activities can ameliorate fatigue because the antioxidant defense gets weaker during fatigue.6 The negative association was found between serum NO and fatigue.37 In addition, the antioxidants have considered as anti-fatigue materials by increasing SOD or CAT levels in liver.23 Our results show that FPP, Lys, and Leu increased SOD and CAT levels and decreased NO level after treadmill exercise. We speculate that FPP, Lys, and Leu would improve the antioxidant defense of the mice after extensive exercise and confer anti-fatigue capability.
In fatigue, BDNF is a kind of neurotrophic factors which play a critical role in brain development, memory, and cognitive performance and known to be an attractive therapeutic target for chronic fatigue.38 A decrease in BDNF expression induces stress in fatigue.39 Our results show that FPP, Lys, and Leu up-regulated the BDNF levels in brain. Therefore, this result indicates that FPP, Lys, and Leu can down-regulated the fatigue through up-regulating the BDNF levels in brain.
Strenuous exercise induces the secretion of the inflammatory cytokines in muscle.40 Pro-inflammatory cytokines were produced by intense physical stress in muscle after strenuous exercise.8 The levels of TNF-α, IL-6, and IL-1β were increased in skeletal muscle and serum in patients with CFS.3,41 The production of pro-inflammatory cytokines was suppressed through blockade of caspase-1 and NF-κB/IκBα pathway in splenocytes.42 Our results show that FPP, Lys, and Leu down-regulated the production of TNF-α, IL-6, IL-1β, and IL-4 as well as the activation of caspase-1 and NF-κB in stimulated splenocyte. Interestingly, FPP, Lys, and Leu up-regulated the production of IFN-γ from stimulated splenocyte. And FPP, Lys, and Leu increased IFN-γ levels in serum and spleen after treadmill exercise. IFN-γ on un-stimulated T cells enhances adaptive immunity by augmenting their survival during the immune response.43 An increase in INF-γ accelerated muscle healing after skeletal muscle injury.44 Thus, we suppose that FPP, Lys, and Leu would have anti-fatigue activity, improving inflammatory reaction through the blockade caspase-1/NF-κB and enhancing immunity by increasing IFN-γ.
Proliferation of naïve T cells from the spleen is promoted in response to anti-CD3 antibody or anti-CD28 antibody.45 T lymphocyte proliferation is associated with stimulation of immune system.46,47 Arginine enhanced the splenocyte proliferation and immune function in an animal model.48 In this study, FPP, Lys, and Leu promoted the splenocyte proliferation without inducing cytotoxicity. Thus, FPP, Lys, and Leu might possess anti-fatigue activity by enhancing immune responses through regulating T lymphocyte proliferation.
Nutritional deficits can be a factor inducing fatigue.49 The porcine placenta contains many prime nutrients regulating stress, cellular protection, and immune function.17 Fermentation has been used to produce more organic acids, amino acids, and vitamins in food or feed applications.50 Lys and Leu contents were the highest among amino acids during the fermentation process of porcine placenta (Table 1). Stress-induced fatigue was associated with a decrease in Lys in blood.51 Leu administration inhibited inflammatory responses and muscle soreness induced by severe exercise.52 In addition, Hou et al.53 reported that fermented placenta inducing bioactive peptides can be developed as ingredients in functional foods and dietary supplements with specific health benefits. Interestingly, porcine placenta extract (0.1 and 1 mg/kg) did not significantly inhibit the immobility times in our previous study,9 whereas in this study, FPP (0.1, 1, and 10 mg/kg) significantly inhibited immobility times. Thus, we suggested that FPP was more effective than porcine placenta extract. However, it is necessary to comparative study about anti-fatigue effects between FPP and porcine placenta extracts.
In conclusion, the present study shows that supplementation with FPP, Lys, and Leu ameliorated fatigue by modulation of fatigue related-biochemical parameters. Therefore, this study signifies the potential of FPP toward anti-fatigue. This study is the first to explore pharmacological effects of FPP on fatigue using treadmill stress and FST models, so further studies will be required to determine.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration Of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
HYK and NRH performed the research and wrote the manuscript. NRK conducted the experiments. ML and JK participated in the design of the study and review of the manuscript. CJK contributed to the treadmill experiments. HJJ and HMK designed the study and analyzed the data.
References
- 1.Uthayathas S, Karuppagounder SS, Tamer SI, Parameshwaran K, Degim T, Suppiramaniam V, Dhanasekaran M. Evaluation of neuroprotective and anti-fatigue effects of sildenafil. Life Sci 2007; 81: 988–92. [DOI] [PubMed] [Google Scholar]
- 2.Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev 2003; 24: 236–52. [DOI] [PubMed] [Google Scholar]
- 3.Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun 2012; 26: 1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coombes JS, McNaughton LR. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J Sport Med Phys Fit 2000; 40: 240–6. [PubMed] [Google Scholar]
- 5.Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chu DW, Brooks GA, Ames BN. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 2000; 89: 21–8. [DOI] [PubMed] [Google Scholar]
- 6.Powers S, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc Nutr Soc 1999; 58: 1025–33. [DOI] [PubMed] [Google Scholar]
- 7.Suárez A, Guillamó E, Roig T, Blázquez A, Alegre J, Bermúdez J, Ventura JL, García-Quintana AM, Comella A, Segura R, Javierre C. Nitric oxide metabolite production during exercise in chronic fatigue syndrome: a case-control study. J Womens Health 2010; 19: 1073–7. [DOI] [PubMed] [Google Scholar]
- 8.Liburt NR, Adams AA, Betancourt A, Horohov DW, McKeever KH. Exercise-induced increases in inflammatory cytokines in muscle and blood of horses. Equine Vet J 2010; 38: 280–8. [DOI] [PubMed] [Google Scholar]
- 9.Han NR, Kim KY, Kim MJ, Kim MH, Kim HM, Jeong HJ. Porcine placenta mitigates protein-energy malnutrition-induced fatigue. Nutrition 2013; 29: 1381–7. [DOI] [PubMed] [Google Scholar]
- 10.Kong MH, Lee EJ, Lee SY, Cho SJ, Hong YS, Park SB. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause 2008; 15: 296–303. [DOI] [PubMed] [Google Scholar]
- 11.Han NR, Park CL, Kim NR, Kim HY, Yoou MS, Nam SY, Moon PD1, Jeong HJ2, Kim HM. Protective effect of porcine placenta in a menopausal ovariectomized mouse. Reproduction 2015; 150: 173–181. [DOI] [PubMed] [Google Scholar]
- 12.Hong HT, Kim HJ, Lee TK, Kim DW, Kim HM, Choo YK, Park YG, Lee YC, Kim CH. Inhibitory effect of a Korean traditional medicine, Honghwain-Jahage (water extracts of Carthamus tinctorius L. Seed and Hominis placenta) on interleukin-1-mediated bone resorption. J Ethnopharmacol 2002; 79: 143–8. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Lee JW, Kim YL, Lee MH. The effect of placental extract on the expression of tyrosinase, TRP-1 and TRP-2 in SK30 melanoma cells. Korean J Dermatol 2003; 41: 1612–8. [Google Scholar]
- 14.Gibbsa BF, Zougmanb A, Massea R, Mulliganc C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res Int 2004; 37: 123–31. [Google Scholar]
- 15.Kang DZ, Hong HD, Kim KI, Choi SY. Anti-Fatigue effects of fermented Rhodiola rosea extract in mice. Prev Nutr Food Sci 2015; 20: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsui Y, Bagchi M, Marone PA, Moriyama H, Bagchi D. Safety and toxicological evaluation of a novel, fermented, peptide-enriched, hydrolyzed swine placenta extract powder. Toxicol Mech Methods 2015; 25: 13–20. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho JF, Masuda MO, Pompeu FA. Method for diagnosis and control of aerobic training in rats based on lactate threshold. Comp Biochem Phys A 2005; 140: 409–13. [DOI] [PubMed] [Google Scholar]
- 18.Al-Malki AL. Suppression of acute pancreatitis by L-lysine in mice. BMC Complement Altern Med 2015; 15: 193–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto T, Nakamura K, Matsumoto H, Sakai R, Kuwahara T, Kadota Y, Kitaura Y, Sato J, Shimomura Y. Bolus ingestion of individual branched-chain amino acids alters plasma amino acid profiles in young healthy men. Springerplus 2014; 17: 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo JH, Sung YH, Kim KJ, Shin MS, Lee EK, Kim CJ. Effects of Phellinus linteus administration on serotonin synthesis in the brain and expression of monocarboxylate transporters in the muscle during exhaustive exercise in rats. J Nutr Sci Vitaminol 2011; 57: 95–103. [DOI] [PubMed] [Google Scholar]
- 21.Jeong HJ, Kim JH, Kim NR, Yoou MS, Nam SY, Kim KY, Choi Y, Jang JB, Kang IC, Baek NI, Kim HM. Antidepressant effect of Stillen. Arch Pharm Res 2015; 38: 1223–31. [DOI] [PubMed] [Google Scholar]
- 22.Rim HK, Kim KY, Moon PD. Evidence of hydrolyzed traditional Korean red ginseng by malted barley on activation of receptor interacting proteins 2 and IkappaB kinase-beta in mouse peritoneal macrophages. TANG 2015; 2: e37–e37. [Google Scholar]
- 23.Sun L, Shen W, Liu Z, Guan S, Liu J, Ding S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: effects of a combination of mitochondrial targeting nutrients. Life Sci 2010; 86: 39–44. [DOI] [PubMed] [Google Scholar]
- 24.Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M, Kim S, Raja SM, Shi L, Simon MM, Froelich CJ. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 2008; 29: 720–33. [DOI] [PubMed] [Google Scholar]
- 25.Jeong HJ, Chung HS, Lee BR, Kim SJ, Yoo SJ, Hong SH, Kim HM. Expression of proinflammatory cytokines via HIF-1alpha and NF-kappaB activation on desferrioxamine stimulated HMC-1 cells. Biochem Biophys Res Commun 2003; 306: 805–11. [DOI] [PubMed] [Google Scholar]
- 26.Ament W, Verkerke GJ. Exercise and fatigue. Sports Med 2009; 39: 389–422. [DOI] [PubMed] [Google Scholar]
- 27.Chang Q, Miao X, Ju X, Zhu L, Huang C, Huang T, Zuo X, Gao C. Effects of pulse current on endurance exercise and its anti-fatigue properties in the hepatic tissue of trained rats. PLoS One 2013; 8: e75093–e75093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brancaccio P, Limongelli FM, Maffulli N. Monitoring of serum enzymes in sport. Br J Sports Med 2006; 40: 96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing LJ, Cui GW, Feng Q, Xiao YS. Orthogonal test design for optimization of the extraction of polysaccharides from Lycium barbarum and evaluation of its anti-athletic fatigue activity. J Med Plant Res 2009; 3: 433–7. [Google Scholar]
- 30.Kratz A, Lewandrowski KB, Siegel AJ, Chun KY, Flood JG, Van Cott EM, Lee-Lewandrowski E. Effect of marathon running on hematologic and biochemical laboratory parameters, including cardiac markers. Am J Clin Pathol 2002; 118: 856–63. [DOI] [PubMed] [Google Scholar]
- 31.Powell J, DiLeo T, Roberge R, Coca A, Kim JH. Salivary and serum cortisol levels during recovery from intense exercise and prolonged, moderate exercise. Biol Sport 2015; 32: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001; 17: 107–24. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Wang J, Shao JQ, Du H, Wang YT, Peng L. Effect of Schisandra chinensis on interleukins, glucose metabolism, and pituitary-adrenal and gonadal axis in rats under strenuous swimming exercise. Chin J Integr Med 2015; 21: 43–8. [DOI] [PubMed] [Google Scholar]
- 34.Roach PJ, Skurat AV. Harris RA. The endocrine pancreas and regulation of metabolism. 1st ed. New York, NY: Oxford University Press, 2001.
- 35.Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL, DePaoli-Roach AA, Roach PJ. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem 2005; 280: 17260–5. [DOI] [PubMed] [Google Scholar]
- 36.Ren J, Zhao M, Wang H, Cui C, You L. Effects of supplementation with grass carp protein versus peptide on swimming endurance in mice. Nutrition 2011; 27: 789–95. [DOI] [PubMed] [Google Scholar]
- 37.Takaki J. Associations of job stress indicators with oxidative biomarkers in Japanese men and women. Int J Environ Res Public Health 2013; 10: 6662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saligan LN, Lukkahatai N, Holder G, Walitt B, Machado-Vieira R. Lower brain-derived neurotrophic factor levels associated with worsening fatigue in prostate cancer patients during repeated stress from radiation therapy. World J Biol Psychiatry 2015; 27: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 2007; 18: 391–418. [DOI] [PubMed] [Google Scholar]
- 40.Petersen AM, Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. Acute moderate elevation of TNF-alpha does not affect systemic and skeletal muscle protein turnover in healthy humans. J Clin Endocrinol Metab 2009; 94: 294–9. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Trans Med 2009; 7: 96–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 2010; 185: 4385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed JM, Branigan PJ, Bamezai A. Interferon gamma enhances clonal expansion and survival of CD4+ T cells. J Interferon Cytokine Res 2008; 28: 611–22. [DOI] [PubMed] [Google Scholar]
- 44.Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Phys Cell Phys 2008; 294: C1183–91. [DOI] [PubMed] [Google Scholar]
- 45.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest 2002; 109: 295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford DH, Chen S, Boyd CA. Cationic amino acid transport in human T lymphocytes is markedly increased in the CD45RA, CD8+ population after activation. Immunology 1994; 82: 357–600. [PMC free article] [PubMed] [Google Scholar]
- 47.Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E. Effects of L-arginine on the proliferation of T lymphocyte subpopulations. J Parenter Enteral Nutr 2001; 25: 23–9. [DOI] [PubMed] [Google Scholar]
- 48.Suarez Butler MF, Langkamp-Henken B, Herrlinger-Garcia KA, Klash AE, Szczepanik ME, Nieves C, Jr, Cottey RJ, Bender BS. Arginine supplementation enhances mitogen-induced splenocyte proliferation but does not affect in vivo indicators of antigen-specific immunity in mice. J Nutr 2005; 135: 1146–50. [DOI] [PubMed] [Google Scholar]
- 49.Shephard RJ. Chronic fatigue syndrome. A brief review of functional disturbances and potential therapy. J Sports Med Phys Fitness 2005; 45: 381–92. [PubMed] [Google Scholar]
- 50.Chen Y, Nielsen J. Biobased organic acids production by metabolically engineered microorganisms. Curr Opin Biotechnol 2015; 37: 165–72. [DOI] [PubMed] [Google Scholar]
- 51.Mizuno K, Tanaka M, Nozaki S, Yamaguti K, Mizuma H, Sasabe T, Sugino T, Shirai T, Kataoka Y, Kajimoto Y, Kuratsune H, Kajimoto O, Watanabe Y. Mental fatigue-induced decrease in levels of several plasma amino acids. J Neural Transm 2007; 114: 555–61. [DOI] [PubMed] [Google Scholar]
- 52.Cruzat VF, Krause M, Newsholme P. Amino acid supplementation and impact on immune function in the context of exercise. J Int Soc Sports Nutr 2014; 11: 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou Y, Zhou J, Liu W, Cheng Y, Wu L, Zhu X, Bai Y, Yang G. Fermentation process and bioactivity of peptides prepared from fermented goat placenta residues. Trans Chin Soc Agric Eng 2015; 31: 311–6. [Google Scholar]