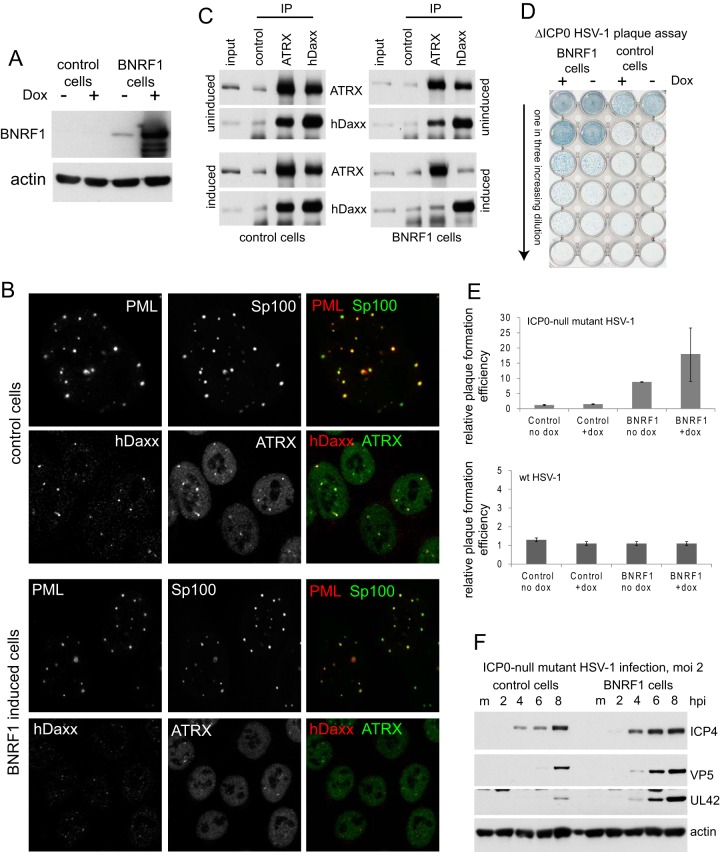
FIG 1.
BNRF1 increases the replication efficiency of ICP0-null mutant HSV-1. (A) Inducible expression of BNRF1 in HepaRG-based cells after treatment with 100 ng/ml doxycycline (Dox) for 24 h. (B) Induction of BNRF1 expression causes the displacement of hDaxx and ATRX, but not Sp100, from PML NBs. Each row of three images shows the separated and merged channels for the relevant protein, as indicated. The top two rows show control HA-TetR cells; the lower pair show induced BNRF1-expressing cells. (C) Induction of BNRF1 expression causes the disruption of the hDaxx/ATRX complex. Extracts made from uninduced and induced control and BNRF1-expressing cells were used for immunoprecipitation (IP) with control, hDaxx, or ATRX antibodies, as marked. Substantial coimmunoprecipitation of hDaxx and ATRX occurred in the control extracts, but in BNRF1-expressing cells this was reduced to antibody control levels. (D) Expression of BNRF1 increases ICP0-null mutant plaque formation. Virus dl1403/CMVlacZ was used to infect uninduced and induced control and BNRF1-expressing cells at increasing 3-fold dilutions. Plaques were visualized by staining for the β-galactosidase marker gene at 24 h after infection. (E) Quantification of plaque formation efficiencies from replicate experiments of the type shown in panel D. Relative plaque-forming efficiency was calculated by comparing the number of plaques under the stated condition with that of the same input virus dilution. (F) Expression of BNRF1 increases the efficiency of viral gene expression in ICP0-null mutant HSV-1 infections. Induced control and BNRF1 cells were infected at an MOI of 2, and then samples taken at the indicated times after infection were analyzed for ICP4, VP5, and UL42 expression. hpi, hours postinfection.