FIG 3.
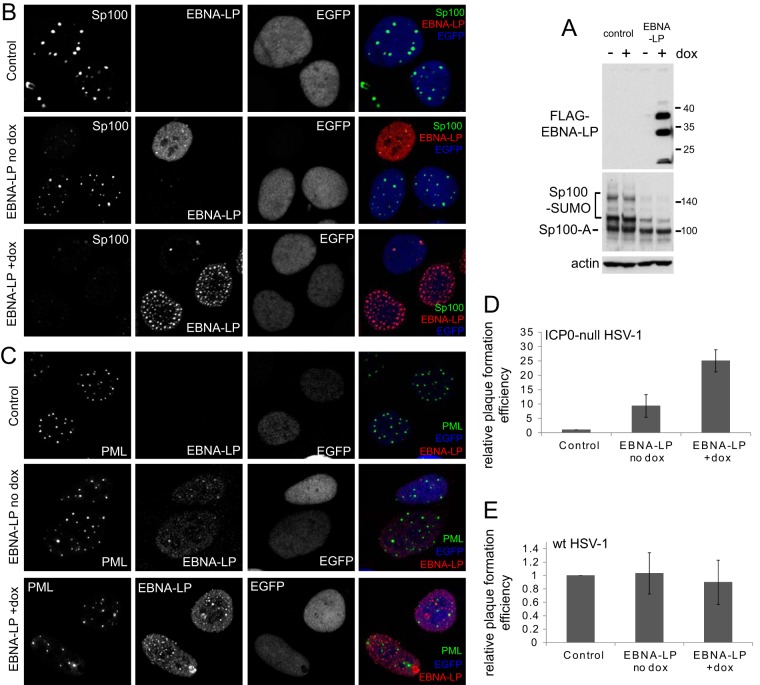
EBNA-LP increases the replication efficiency of ICP0-null mutant HSV-1. (A) Inducible expression of EBNA-LP in HepaRG-based cells after treatment with 100 ng/ml doxycycline (Dox) for 24 h. Analysis of Sp100 indicated substantial loss of the sumoylated forms of this protein in the BNRF1-expressing cells. (B) Induction of EBNA-LP expression causes the loss of Sp100 from PML NBs. Control, uninduced, and induced HFT EBNA-LP-expressing cells were stained for Sp100 and EBNA-LP as indicated. EGFP detects the nuclear localization of the EGFPnlsTetR fusion protein in these cells. A proportion of uninduced cells, particularly those expressing a low level of the TetR repressor, have detectable EBNA-LP expression, and in these cells Sp100 staining is much reduced. This phenotype is widespread in induced cells. (C) Cells as described in panel B were stained for PML and EBNA-LP. PML staining was unaffected by expression of EBNA-LP, whose punctate foci largely colocalized with PML in uninduced cells and to a lesser extent in induced cells. (D) Induction of EBNA-LP expression increases ICP0-null mutant plaque formation. Virus dl1403/CMVlacZ was used to infect uninduced control and EBNA-LP-expressing cells at increasing 3-fold dilutions, and then relative plaque-forming efficiencies were calculated by comparing the numbers of plaques in the two cell types with the same input virus dilutions. (E) EBNA-LP expression has no effect on the plaque formation efficiency of wt HSV-1.