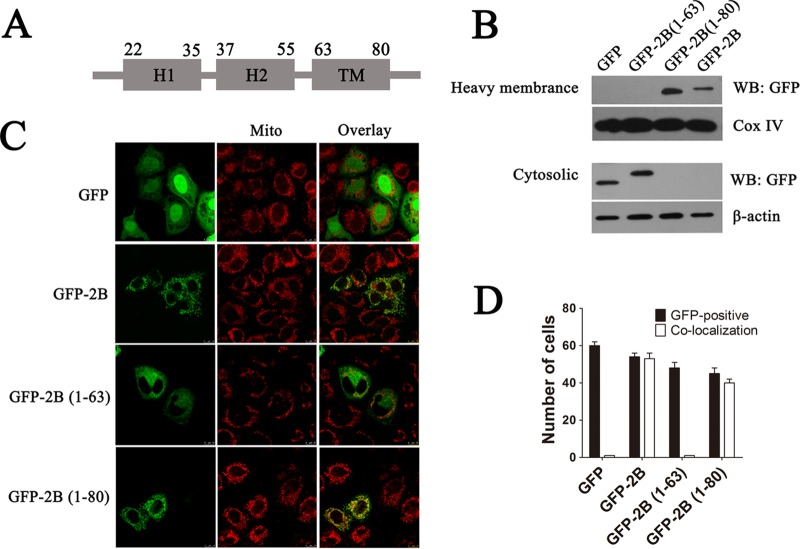
FIG 8.
Identification of regions that are responsible for targeting 2B to the mitochondrial outer membrane. (A) Schematic diagram of 2B indicating the following: a transmembrane region (TM), a putative C-terminal hydrophobic α-helix (aa 63 to 80); H2, an amphipathic α-helix (aa 37 to 55); and H1, an N-terminal hydrophilic helix (aa 22 to 35). The secondary structure and helix of 2B were predicted by using the Predict Protein and TMHMM server. (B) HeLa cells were transfected with pGFP-2B(1–63) (GFP fused to aa 1 to 63 of 2B), pGFP-2B(1–80) (GFP fused to aa 1 to 80 of 2B), or pGFP-2B for 24 h. The cytosol and mitochondrial fractions were separated and immunoblotted with GFP antibody. Cox IV and β-actin were used as the internal controls for the mitochondrial and cytosol fractions, respectively. (C) Confocal microscopy analysis of the colocalization of pGFP-2B(1–80) and pGFP-2B with the mitochondria. Cells were transfected with pGFP, pGFP-2B(1–63), pGFP-2B(1–80), or pGFP-2B as described above. At 24 h posttransfection, the cells were incubated with MitoTracker Red (red) for 10 min, fixed, and subjected to confocal microscopy analysis. (D) Colocalization indexes for cells transfected with pGFP, pGFP-2B, pGFP-2B(1–63), or pGFP-2B(1–80) and labeled for MitoTracker Red as for panel C. Cells with GFP and mitochondrial colocalization (Manders overlap coefficient of >0.8) were counted as described for Fig. 1.