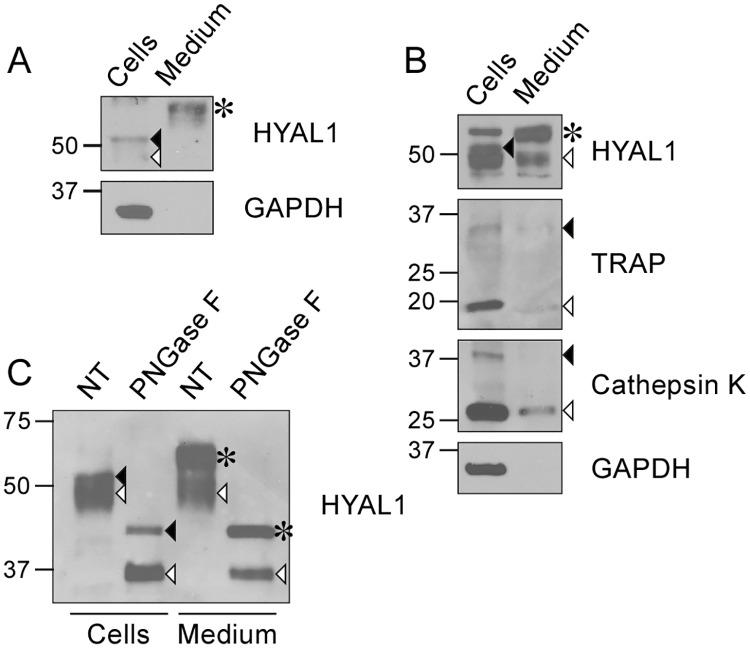
Fig 5. Osteoclasts secrete HYAL1 through both the constitutive secretory pathway and lysosomal exocytosis.
(A) Detection of HYAL1 by western blotting, under reducing conditions, in RAW264.7 cell lysate and medium samples collected after a 5 h incubation in serum-free conditions. The cytosolic protein GAPDH was used as a control of the cell integrity. The medium was concentrated 4-fold compared to the cell sample. The closed arrowhead, open arrowhead and asterisk mark the 52 kDa intracellular precursor form, 48 kDa intracellular mature form and 65 kDa secreted form of HYAL1, respectively. (B) Detection of cathepsin K, TRAP, GAPDH and HYAL1 by western blotting in RAW264.7-derived osteoclast cell lysate and concentrated medium (4 x). The precursor and mature forms of TRAP and cathepsin K are highlighted by closed and open arrowheads, respectively. The different forms of HYAL1 are marked as described in A. (C) Aliquots of osteoclast cell lysate and medium (concentrated 4-fold) were treated with PNGase F prior to the detection of HYAL1 by western blotting. The different forms of HYAL1 are pointed as described in A. Note that the extracellular precursor form (asterisk) exhibited the same MM as the intracellular precursor (closed arrowhead) after deglycosylation, and that osteoclasts secreted mature forms of HYAL1 (detected at ~35 kDa after deglycosylation, open arrowhead).