Figure 1.
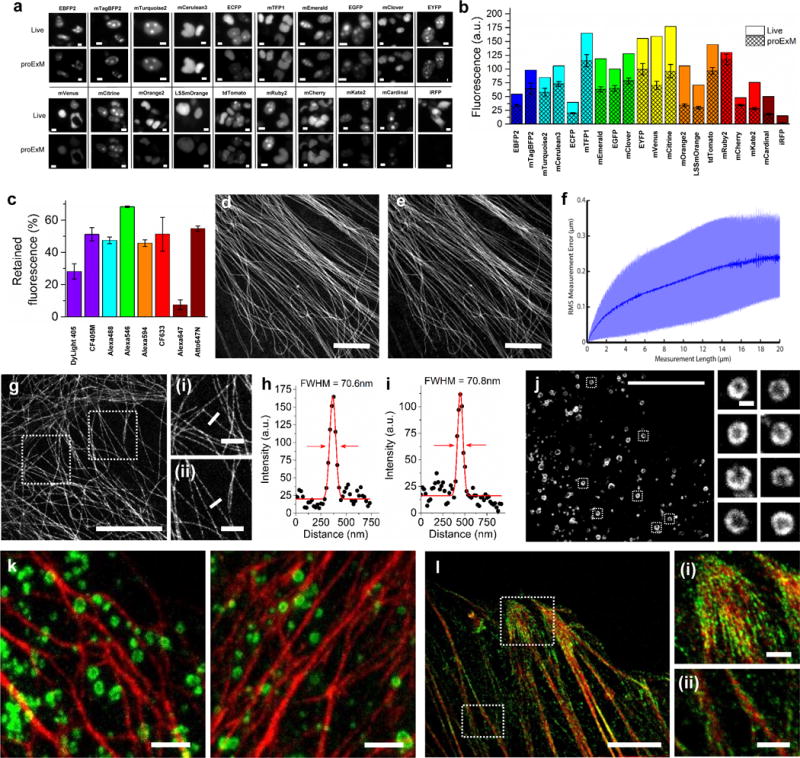
Retention of fluorescent protein (FP) and antibody fluorescence signals in proExM and proExM of FP fusions. (a) Representative images of selected FP-histone fusion proteins in live HEK293FT cells (upper row) and in the same cells after proExM treatment (lower row); iRFP was expressed as N-terminal fusion with nuclear localization sequence (NLS). (b) Quantified fluorescence of experiments as in panel a, after proExM treatment (crosshatched bars; mean ± standard deviation; n = 4 transfection replicates each). Open bars, literature values of the brightnesses of these fluorophores, normalized to the brightness of EGFP. (c) Retention of fluorescence for selected dyes conjugated with antibodies, after proExM treatment (mean ± standard deviation, n = 3 samples each), in mouse brain slice. (d) Super-resolution structured illumination microscopy (SR-SIM) image of immunostained microtubules after the anchoring step vs. (e) post-expansion image of the same sample acquired with a spinning disk confocal microscope. (f) Root mean square (RMS) length measurement error as a function of measurement length for proExM vs SIM images (blue line, mean; shaded area, standard deviation; n = 4 samples). (g) Confocal image of mClover-α-tubulin fusion. HeLa cells are used throughout the rest of this figure. Panels (i and ii) are magnified views of boxed regions in (g). Linecuts are quantified in panels h, i. Solid red lines in (h, i) indicate the Gaussian fit used to determine the full width at half maximum (FWHM; illustrated with red arrows). (j) Confocal image of mEmerald-clathrin fusion (left) and magnified views of single CCPs in the boxed regions (right). (k) Dual color proExM of clathrin (fused to mEmerald, green) and keratin (mRuby2, red). (l) Dual color proExM image of actin (mRuby2, red) and paxillin (mEmerald, green) fusions. Panels (i and ii) are magnified views of boxed regions in (f). Scale bars: (a) 5 μm, (d) 5 μm, (e) 5 μm (physical size post-expansion, 20.5 μm), (g) 5 μm (21.5 μm), (i–ii) 1 μm; (j) 10 μm (42.6 μm), insets 200 nm; (k) 1 μm (4.3 μm), (l) 5 μm (21.5 μm), (i–ii) 1 μm.
