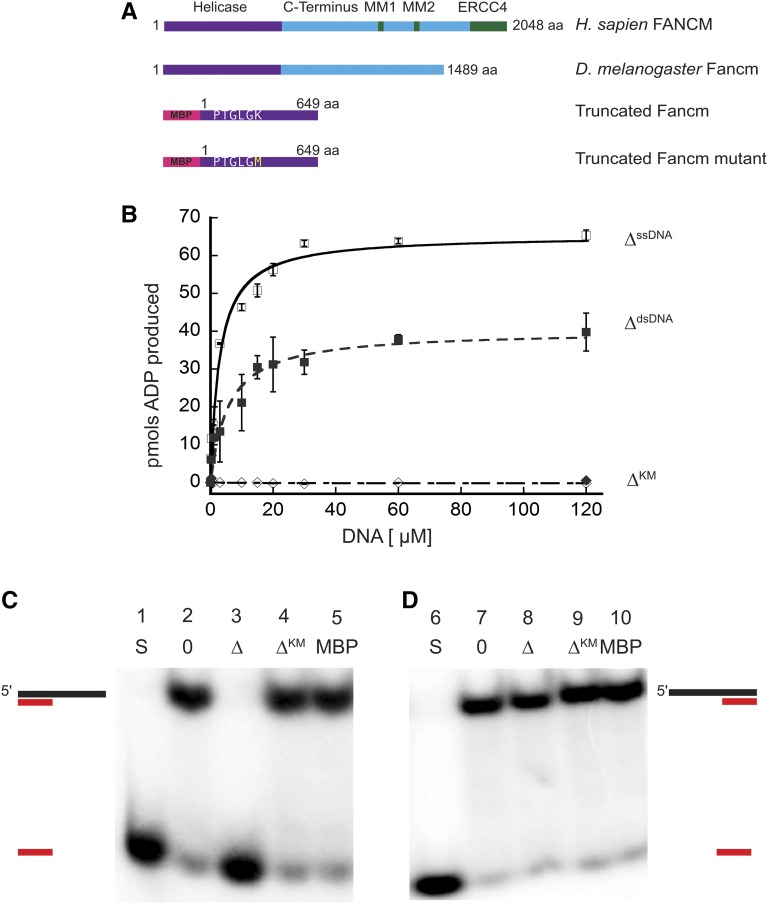
Figure 1.
Drosophila Fancm is a 3′ to 5′ DNA helicase dependent on ATP hydrolysis. (A) Schematic of Fancm. Domains and motifs present in human FANCM are marked. Conserved domains or motifs in D. melanogaster are noted. Truncated forms depicted are with an N-terminal MBP tag. (B) ATP hydrolysis by Fancm. Fancm ATPase activity was examined as a function of DNA concentration using either M13mp18 ssDNA (□⋄) or dsDNA (▪♦) as the DNA cofactor. All reactions were incubated at 37° for 5 min. □ ▪ 212 nM Fancm∆ on ssDNA (∆ssDNA); ⋄♦ 212 nM Fancm∆KM (∆KM). The average values from at least three independent experiments were plotted. Error bars represent SEM (ssDNA) or SD of the mean (dsDNA). (C) Fancm unwinds duplex DNA. Protein (212 nM) was incubated with a 5′ radiolabeled 15-bp partial duplex with a 25-nt 3′ overhang (15/40). (D) Fancm is a 3′ to 5′ DNA helicase. Protein (212 nM) was incubated with a 5′ radiolabeled 15-bp partial duplex with a 25-nt 5′ overhang (−15/40). Lane 1 and 6 (S) are boiled loading controls indicating ssDNA. Lanes 2 and 7 (0) are no-protein controls. Fancm∆ in lane 3 and 8 (∆), Fancm∆KM in lane 4 and 9 (∆KM), and MBP in lane 5 and 10 (MBP). Colored strand represents radiolabeled strand. Substrate oligonucleotides are in Table S1.