Abstract
Protective mechanisms based on RNA silencing directed against the propagation of transposable elements are highly conserved in eukaryotes. The control of transposable elements is mediated by small noncoding RNAs, which derive from transposon-rich heterochromatic regions that function as small RNA-generating loci. These clusters are transcribed and the precursor transcripts are processed to generate Piwi-interacting RNAs (piRNAs) and endogenous small interfering RNAs (endo-siRNAs), which silence transposable elements in gonads and somatic tissues. The flamenco locus is a Drosophila melanogaster small RNA cluster that controls gypsy and other transposable elements, and has played an important role in understanding how small noncoding RNAs repress transposable elements. In this study, we describe a cosuppression mechanism triggered by new euchromatic gypsy insertions in genetic backgrounds carrying flamenco alleles defective in gypsy suppression. We found that the silencing of gypsy is accompanied by the silencing of other transposons regulated by flamenco, and of specific flamenco sequences from which small RNAs against gypsy originate. This cosuppression mechanism seems to depend on a post-transcriptional regulation that involves both endo-siRNA and piRNA pathways and is associated with the occurrence of developmental defects. In conclusion, we propose that new gypsy euchromatic insertions trigger a post-transcriptional silencing of gypsy sense and antisense sequences, which modifies the flamenco activity. This cosuppression mechanism interferes with some developmental processes, presumably by influencing the expression of specific genes.
Keywords: transposon, small RNA, RNA silencing, primary transcript, ecdysis
EUKARYOTIC genomes consist in part of sequences derived from a wide variety of transposable elements (TEs), some of which can mobilize to new genomic locations (de Koning et al. 2011). A genomic consequence of their mobilization is the induction of new mutations and chromosomal rearrangements that may have deleterious effects on fitness. However, they may also provide a fundamental contribution to genetic variation and evolutionary changes (Fedoroff 2012; Warren et al. 2015; Elbarbary et al. 2016; Mita and Boeke 2016).
TE activation is suppressed by specific silencing mechanisms that act both at the transcriptional level, through chromatin modifications, and at the post-transcriptional level (Buchon and Vaury 2006). Piwi-interacting RNAs (piRNAs), a distinct class of 24- to 30-nt-long RNAs produced by a Dicer-independent biogenesis pathway, are involved in the recognition and selective silencing of transposons during gametogenesis (Sarot et al. 2004; Kalmykova et al. 2005). In the Drosophila ovary germline, the coordinated action of aubergine (aub), Argonaute 3 (AGO3), and piwi suppresses activity of a broad group of TEs through the formation of piRNAs involving both a primary processing and a secondary “ping-pong” amplification loop (Brennecke et al. 2007; Gunawardane et al. 2007; Li et al. 2009; Malone et al. 2009), while in the follicular epithelium a different mechanism exists with piRNAs generated solely from primary transcript derived mainly from the flamenco locus. Somatic activation of TEs is mainly controlled by endogenous small interfering RNAs (endo-siRNAs), generated by Dicer-2 (Dcr-2) and Argonaute 2 (AGO2) genes (Czech et al. 2008; Ghildiyal et al. 2008). Endo-siRNAs can arise from genomic loci from which piRNAs also originate.
The flamenco locus, also known as the COM locus, is a major regulator of the gypsy retrotransposon and other TEs, such as ZAM and Idefix (Pélisson et al. 1994; Desset et al. 1999; Desset et al. 2003; Goriaux et al. 2014b). gypsy is repressed in the follicular epithelium of flamenco restrictive females, while it is active and able to propagate within the germline in permissive backgrounds (Pélisson et al. 1994; Bucheton 1995; Song et al. 1997; Chalvet et al. 1999). It is reported that natural populations of Drosophila melanogaster exhibit a restrictive/permissive polymorphism for flamenco (Pélisson et al. 1997). The flamenco locus is a uni-strand cluster comprising a region of ∼180 kb, located in the pericentromeric heterochromatin of the X chromosome at 20A1–A3. The sequence of flamenco mostly consists of a high density of antisense transposon fragments, the majority of which belong to the LTR group of retrotransposons (Brennecke et al. 2007; Mevel-Ninio et al. 2007). The flamenco locus can be considered a small RNA cluster since in the ovary it acts as a source of piRNAs, while in somatic tissues outside of the ovary it acts as a source of endo-siRNAs (Brennecke et al. 2007; Ghildiyal et al. 2008). The role of flamenco is to prevent the expression of gypsy and other TEs having complementary sequences in this locus, and for this reason it is considered part of the “adaptive immunity” for transposon control (Bergman et al. 2006). The ability to control TE transposition is variable and seems to be associated with the high structural dynamics of the flamenco locus, which depends on chromosomal rearrangements and loss or gain of TEs inside the locus (Zanni et al. 2013).
Important information regarding the mechanism by which small noncoding RNAs repress TEs derive from the study of the flamenco locus. However, still little is known about flamenco regulation. It is known that unidirectional transcription of flamenco, which is transcribed from an RNA polymerase II promoter located ∼2 kb upstream of the DIP1 gene and activated by the Cubitus interruptus transcription factor, produces a long precursor transcript (Goriaux et al. 2014a). The flamenco precursor transcript is then subjected to alternative splicing to produce RNA precursors that are then processed to piRNAs. It is also known that small RNA clusters like flamenco are subjected to heterochromatin silencing and there is evidence that their expression can be modified by mutations affecting heterochromatin formation (Moshkovich and Lei 2010).
In this study, we analyzed the effect of some euchromatic gypsy mutations in Drosophila strains carrying flamenco alleles permissive for gypsy mobilization. Besides the known phenotypes due to the gypsy insertion in the specific locus, we found additional phenotypes depending on a genetic interaction between the specific euchromatic gypsy insertion and the flamenco permissive allele. To characterize this genetic interaction, the experiments were mainly performed using gypsy-induced mutations directly isolated from a flamenco permissive strain, using the original strain as a control. In this way, we detected variations in phenotypes and in expression levels in flies with a common genetic background. This minimized the possibility of detecting background effects. We found that these phenotypes are accompanied by cosuppression of both gypsy retrotransposons and gypsy antisense fragments that are part of the flamenco primary transcript. The cosuppression mechanism seems to be triggered by the gypsy euchromatic insertion site, which shows specific characteristics of new small RNA clusters. Cosuppression of gypsy and flamenco depends on an RNA silencing mechanism which is not accompanied by an increase of the heterochromatic marks H3K9me3 and H3K27me3 at the flamenco locus, at gypsy sequences, as well as at the other TEs analyzed. These data suggest that the described cosuppression mechanism occurs at post-transcriptional level.
Materials and Methods
Drosophila stocks
Drosophila stocks were maintained at 25° on standard cornmeal/yeast medium. y w67c23 and Canton-S have been used as wild-type strains. AstC-R2f03116, ct6, ctn, Su(Hw)8, Bx2, flamGB02658, AGO3t2, Su(var)3-7DG10405, Su(var)3-91, Su(var)3-92, Su(var)2055, f1, and Df(1)l11/FM7c, flamFM7c; P{gypsy-lacZ.p12} were obtained from Bloomington Stock Center. flam1 and flamsu(f) were kindly provided by Alain Pélisson. Since AstC-R2f03116 flies were positive for Wolbachia, they were treated for two generations with high-dose tetracycline (0.25 mg/ml) to remove the pathogen. To eliminate viruses and other horizontally transmitted pathogens, AstC-R2f03116 eggs were dechlorinated in 50% bleach for 2 min. Flies carrying the gypsy-lacZ transgene were obtained by crossing Df(1)l11/FM7c, flamFM7c; P{gypsy-lacZ.p12} females with w flamA, w ctA flamA, or flamsu(f) males. w flamA/flam1; P{gypsy-lacZ.p12}/+ flies were obtained by crossing w flamA; P{gypsy-lacZ.p12} females with flam1 males. ct6 flam1, ctn flam1, and Bx2 flam1 flies were obtained by recombination, by selecting ct and Bx phenotypes and the eye phenotype induced by the mal1 mutation which maps very close to the flam1 mutation. Different independent lines were analyzed to confirm the phenotype. The ctA flamBG line was obtained by recombination, by selecting the ct phenotype and variegated eye color due to the presence of the mini-white gene carried by the P element inserted in the flamenco locus. Genetic combinations between w flamA or w ctA flamA and Su(Hw)8, Su(var)3-7DG10405, Su(var)3-91, Su(var)3-92, AGO2414, and AGO3t2 were obtained by crossing w flamA or w ctA flamA females carrying the TM6b/TM3 Sb balancer combination to males carrying the specific balanced mutations. Genetic combinations between w flamA or w ctA flamA and Su(var)2055, Dcr-2L811fsX, and aubHN2 were obtained by crossing w ctA flamA; CyO/Sp females with males carrying the specific balanced mutations. All experiments with flamenco permissive alleles, except for flamGB, were performed using females homozygous for the X chromosome carrying the flamenco allele.
Mortality determination
For calculation of mortality during adult ecdysis, parent flies were allowed to lay eggs in standard vials for 3–4 days. Eclosed adults and adults that died within the puparium were then counted, and the percentage of mortality was calculated. Three separate experiments were carried out counting at least 100 individuals per experiment, and the average and SD were calculated. P value was calculated using the two-tailed unpaired Student’s t-test (** P < 0.01).
β-Galactosidase staining
Ovaries were dissected in PBS, transferred and fixed in PBS plus Triton X-100 (PBT) (PBS with 0.1% Triton X-100) containing 0.1% glutaraldehyde for 5 min, and rinsed three times with PBT. Each ovary sample was then incubated with 0.2% X-gal in staining solution {10 mM phosphate buffer, pH 7.2, 1 mM MgCl2, 5 mM K4[FeII(CN)6], 5 mM K3[Fe(III)(CN)6], 0.1% Triton X-100} for 45 min. After staining, ovary samples were rinsed three times with PBT, mounted in PBS containing 50% glycerol, and examined by bright-field microscopy.
Microscopic analysis
Living pupae, pharate adults extracted from cocoons, and adult eyes were analyzed and photographed by bright-field stereoscopic microscopy. Adult wings were dissected and dehydrated in ethanol, mounted in a solution of lactic acid:ethanol (6:5), and examined by bright-field microscopy.
Quantitative RT-PCR
Total RNA was extracted by crushing 15–20 pupae (48 and 96 hr), 20 ovaries (3 days old), and 50–100 heads (0- to 24-hr-old females) in TRI Reagent (Sigma-Aldrich) and subsequently treated with TURBO DNase (Ambion). Complementary DNAs (cDNAs) were prepared using M-MLV Reverse Transcriptase (Ambion) and random primers, according to the manufacture’s protocol. To analyze strand-specific expression, cDNAs were produced using strand-specific primers. Real-time PCRs were performed in Fast 96-well Reaction Plates (Applied Biosystems, Foster City, CA) using an Applied Biosystems 7900 HT Fast Real-Time PCR System according to the manufacturer’s suggested procedure. After the PCR run, dissociation curve analysis was performed to determine target specificity. Three replicates were used for each experimental sample. Messenger RNA (mRNA) levels were normalized to the internal standard gene Rp49. To measure the fold change of expression levels between control and experiment(s), the ΔΔCt method was used. Three biological replicates were used and the average and SD were calculated. Differences between experiments and controls were tested with the one-sample Student’s t-test (* P < 0.05, ** P < 0.01, *** P < 0.005). For quantitative PCR (qPCR) primers see Supplemental Material, Table S1.
Chromatin immunoprecipitation
Chromatin preparation and immunoprecipitation was carried out as previously described (Schauer et al. 2013), with some modifications. Heads of frozen flies were separated using 630 and 400 μm sieves. Then, 800–1000 frozen fly heads were ground to a fine powder with a mortar and pestle (chilled on dry ice), resuspended in 25 ml ice-cold homogenization buffer [350 mM sucrose, 15 mM HEPES, pH 7.6, 10 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 0.1 mM EDTA, 0.1% Tween, with 1 mM DTT and Hoffman La Roche (Nutley, NJ) Protease Inhibitor Cocktail added immediately prior to use], incubated for 5 min on ice, and then further ground in a mechanical homogenizer (rotation 2000 rpm, 20 ups and downs, slowly). After centrifugation (5 min, 2000 × g, 4°), the homogenate was resuspended in 25 ml room-temperature homogenization buffer, fixed using 1% formaldehyde for 10 min at room temperature, and then quenched for 5 min with 0.125 M glycine. The tissue debris was removed by filtration with a 70-μm nylon cell strainer (BD Falcon) and nuclei were collected by centrifugation (5 min, 2000 × g, 4°). Nuclei were resuspended in 1 ml ice-cold radio immunoprecipitation assay (RIPA) buffer [150 mM NaCl, 25 mM HEPES, pH 7.6, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate (DOC), with protease inhibitors added prior to use], transferred to a 1.5-ml low-binding tube, and washed twice with 1 ml ice-cold RIPA. Nuclei were collected by centrifugation (1 min, 3500 × g). Finally, the nuclei were resuspended in 1 ml Shearing Buffer (150 mM NaCl, 25 mM HEPES, pH 7.6, 1 mM EDTA, 0.3% SDS, 0.1% DOC, with protease inhibitors added prior to use) and transferred to 1 ml Covaris Adaptive Focused Acoustics tubes (catalogue number: 520081). Chromatin was fragmented to an average size of 100–400 bp in a Covaris S220 device (30 min; 4°; peak incident power, 120; duty factor, 20; cycles per burst, 200). After shearing, the debris was collected by centrifugation (10 min, 16,000 × g, 4°), the chromatin was transferred to a low-binding tube, and stored at −80°. Fragment size was checked after cross-link reversal on a 1% agarose gel. A 150 μl volume of chromatin (corresponding to ∼150 heads) was used for each chromatin immunoprecipitation (ChIP) assay. For each ChIP assay, 30 μl Dynabeads Protein G (Invitrogen, Carlsbad, CA) was washed with ice-cold PBS 0.1% Tween 20 (PBST) and incubated in 500 µl PBST with the appropriate antibody (6 μl anti-H3K9me3, Active Motif, catalogue number 39161; 6 μg anti-H3K27me3, Diagenode, catalogue number 069-050) for 2 hr on a rotating wheel (10 rpm) at 4°. After washing with PBST, the Protein G beads prebound with or without (negative control) the antibody were resuspended in 850 µl ice-cold RIPA buffer, added to chromatin, and left in rotation (10 rpm) at 4° for 16 hr. After immunopurification, beads were washed (each wash 10 min, 20 rpm at 4°) four times with 1 ml RIPA, once with 1 ml LiCl wash buffer (250 mM LiCl, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.5% NP-40, 0.5% DOC), and once with 1 ml TE buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA). Beads were resuspended in 100 µl elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS) and incubated on a Termomixer at 65° and 800 rpm. Beads were removed by a magnetic rack and the cross-link reversal of immunoprecipitated (IP) DNA was carried out overnight at 65° in a PCR machine. Then, 100 µl TE was added, RNA was degraded by 4 μl RNase A (10 mg/ml) for 1 hr at 37° in a Termomixer (600 rpm), and proteins were digested with 4 μl Proteinase K (10 mg/ml) at 55° for 2 hr in a Termomixer (750 rpm). IP DNA was purified using QIAquick PCR Purification columns (QIAGEN, Valencia, CA) and eluted with 50 μl EB Buffer. A volume of 1 μl was used for qPCR analysis. qPCR was performed with the Fast SYBR Green Master Mix qPCR Kit (Applied Biosystems, Foster City, CA) using 10 μl of total reaction, and analyzed on a Light Cycler 480 Real-Time PCR System (Roche Applied Science). Relative quantifications as a percentage of starting material (percentage of input) were determined using the following equation: % (ChIP/total input) = 2^[(Ct(x% input) − log(x%)/log2) − Ct(ChIP)] × 100%. Ct(ChIP) and Ct(x%input) are threshold values obtained from the exponential phase of qPCR for the IP DNA sample and input sample, respectively; (logx%/log2) accounts for the dilution 1:x of the input to balance the difference in amounts of ChIP and input DNA from qPCR. For qPCR primers see Table S1.
Quantification of piRNA expression
Small RNAs were isolated from ∼20 ovaries dissected from 3-day-old females or ∼100 heads from 0- to 24-hr-old females, using the miRVana small RNA Isolation Kit (Ambion) according to the manufacturer’s instructions. RNA was eluted in 100 μl of RNAase-free water and quantified by spectrophotometric analysis. Samples were then diluted to 2 ng/μl. TaqMan Small RNA Assays (Applied Biosystem) was used for the analysis of piRNAs expression. Specific TaqMan primers and probes for piRNA A (5′ TATTGAGCTCACCGAGAAAGGGCTGGC 3′), piRNA B (5′ TGTTGTGAGTGTATCCAGATGGAG 3′), and for the reference 2S ribosomal RNA (Applied Biosystem) were used in quantitative RT-PCR (qRT-PCR). Reverse transcription for piRNA A and B and the reference 2S ribosomal RNA was performed using the TaqMan microRNA Reverse Transcription Kit and the target specific primers provided with the TaqMan small RNA Assay. qPCR was performed using primers and probes provided with the TaqMan small RNA Assay and using TaqMan Universal Master Mix II, no Uracil-N Glycosylase (Applied Biosystems). Real-time PCRs were performed in Fast 96-well Reaction Plates (Applied Biosystems) using an Applied Biosystems 7900 HT Fast Real-Time PCR System according to the manufacturer’s suggested procedure. piRNA levels were normalized to the internal standard 2S ribosomal RNA. To measure the fold change of expression levels between control and experiment(s), the ΔΔCt method was used. Unless otherwise reported, three biological replicates were used and the average and SD were calculated. Differences between experiments and controls were tested with two-tailed one-sample Student’s t-tests (*** P < 0.005).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Drosophila strains are available upon request.
Results
An ecdysis phenotype manifesting during adult eclosion is induced by a gypsy insertion in the cut locus
We explored the identification of a male with a cut-wing phenotype in a strain carrying a P-element insertion in the AstC-R2 gene, which is located in the third chromosome. The cut-wing phenotype was induced by a novel spontaneous mutation affecting the cut locus in the X chromosome, since it did not complement the ct6 allele (Figure 1A). The new ct allele was named ctA. Homozygous mutations in the suppressor of Hairy wing [su(Hw)] gene revert cut-wing phenotypes only when induced by gypsy (Harrison et al. 1993). The wing phenotype induced by the ctA mutation was suppressed by su(Hw)8 (Figure 1A), suggesting that the mutation was effectively induced by gypsy. Indeed a gypsy element was identified in the cut regulatory region (Figure S1, A and B and File S1).This result was not surprising because it is known that the cut locus is a hotspot for gypsy insertions (Jack 1985).
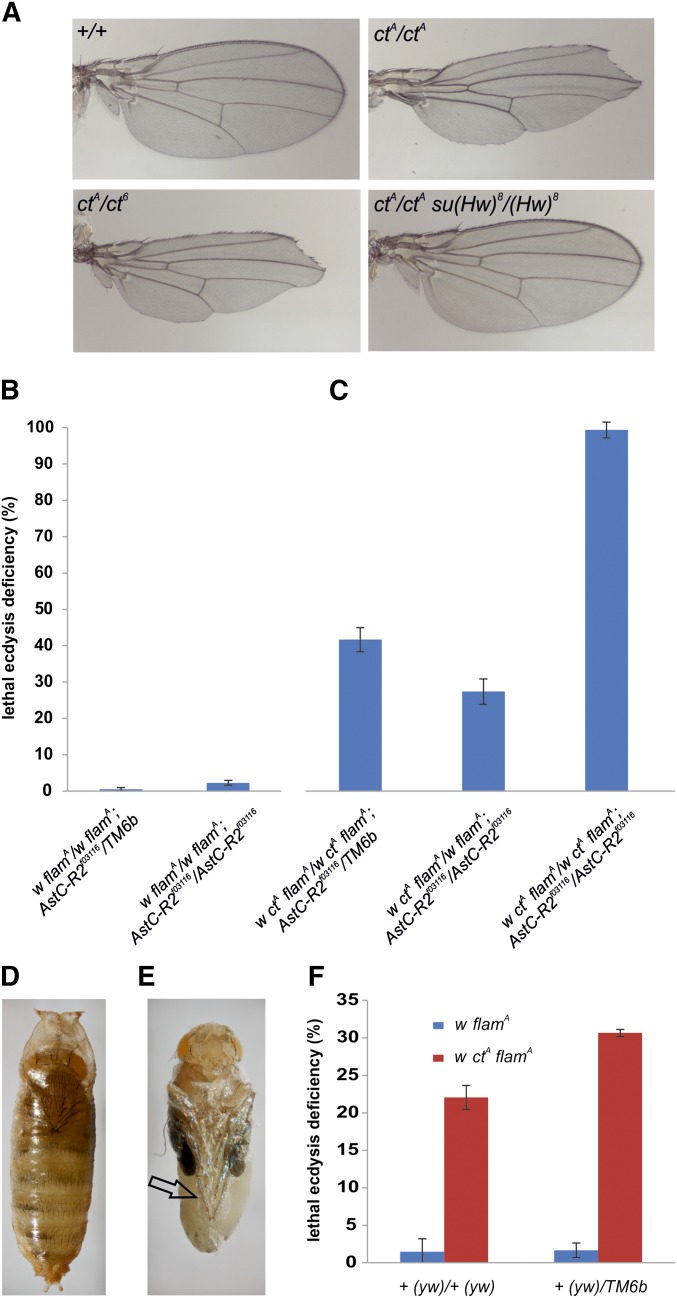
Figure 1.
Lethal ecdysis deficiency is induced by a gypsy insertion in the cut locus. (A) Cuticle preparations of wings from flamA/ flamA (+/+), ctA flamA/ctA flamA (ctA/ctA), ctA flamA/ct6 (ctA/ct6), and ctA flamA/ctA flamA; su(Hw)8/su(Hw)8 (ctA/ctA; su(Hw)8/su(Hw)8). (B, C) Mortality during adult eclosion of flies in the AstC-R2 f03116 background. (B) Mortality during adult eclosion of heterozygous and homozygous flies derived from the mating of AstC-R2 f03116 heterozygous parents. (C) Mortality during adult eclosion of ctA heterozygotes and homozygotes, and AstC-R2 heterozygous and homozygous flies derived from mating of AstC-R2 heterozygous parents. (D, E) representative pictures of ctA/ctA; AstC-R2 f03116/AstC-R2 f03116 pharate adults. (D) Fly trapped inside the puparium ∼12 hr after the start of the adult ecdysis, showing the characteristic pigmentation of eclosed adults. (E) Fly extracted from puparium showing pupal membrane attached to legs, wings, and other parts of the body (arrow). (F) Mortality during adult eclosion of flies with the third chromosomes deriving from the y w67c23 strain (indicated in brackets) in absence or presence of the ctA mutation and of the TM6b balancer chromosome.
A ctA homozygous strain in the AstC-R2f03116 background was obtained (Figure S2A). In addition to the cut-specific phenotypes (Jack 1985), we found that the gypsy mutation induced high levels of mortality during adult eclosion, which was not found in control flies (Figure 1, B and C). In fact, when ctA/ctA flies heterozygous for the AstC-R2f03116 mutation were crossed with each other, we found that ∼40% of the AstC-R2f03116 heterozygous flies (w ctA flamA; AstC-R2f03116/TM6b) and >90% of AstC-R2f03116 homozygous flies (w ctA flamA; AstC-R2f03116/AstC-R2f03116) of the progeny failed to emerge from the puparium (Figure 1C). A significant level of lethal ecdysis deficiency was also observed in flies with only one copy of the ctA allele (Figure 1C, w ctA flamA/w flamA; AstC-R2f03116/AstC-R2f03116 flies), suggesting that one gypsy copy was sufficient to induce the phenotype and that this phenotype does not depend on a cut loss of function. Regarding the phenotype, we found that pharates exhibited characteristic eclosion movements that are common during adult eclosion, but they appeared trapped in the pupal case (Figure 1D). When the pupal case of affected flies was removed, we found that pharates were trapped in the pupal cuticle (Figure 1E).
To exclude that the AstC-R2f03116 mutation was necessary to determine the ecdysis phenotype, the third chromosome carrying the P-element insertion was substituted with a wild-type chromosome from the y w67c23 strain (Figure S2B). The ctA mutation in this wild-type context showed >20% mortality during adult eclosion (Figure 1F), confirming that the gypsy insertion in the cut locus per se induces lethal ecdysis deficiency. Mortality was, however, lower than in AstC-R2f03116 homozygous or heterozygous conditions, suggesting a high sensitivity of this phenotype to the genetic background. In fact, the presence of TM6b in combination with a wild-type third chromosome was sufficient to increase mortality level (Figure 1F), confirming that lethal ecdysis deficiency induced by the gypsy insertion in the cut locus is highly sensitive to the genetic background.
Developmental phenotypes are produced in flamenco permissive backgrounds in the presence of gypsy-induced mutations
Since flamenco permissive alleles are unable to suppress gypsy (Prud’homme et al. 1995), the identification of the gypsy insertion in the cut locus suggested that ctA flies carry a flamenco permissive allele. flamenco permissivity can be evaluated by monitoring the activity of transgenes carrying fragments of gypsy fused to the lacZ reporter (Sarot et al. 2004). We observed strong activation of the gypsy-lacZ construct when the original X chromosome from the AstC-R2f03116 strain was combined with an X chromosome carrying the Df(1)l11 deficiency (Figure 2, A and B), which encompasses the flamenco locus (Prud’homme et al. 1995; Pélisson et al. 1997). flamenco restrictive alleles are dominant and repress the activation of gypsy even when in combination with permissive alleles (Pélisson et al. 1997). The X chromosome carrying the flamA allele combined with an X chromosome carrying the flamFM7c restrictive allele repressed the gypsy-lacZ construct (Figure 2C), while combined with the permissive flam1 allele activated the expression of gypsy-lacZ (Figure 2D). Based on these results, the flamenco permissive allele in the ctA-carrying chromosome was named flamA.
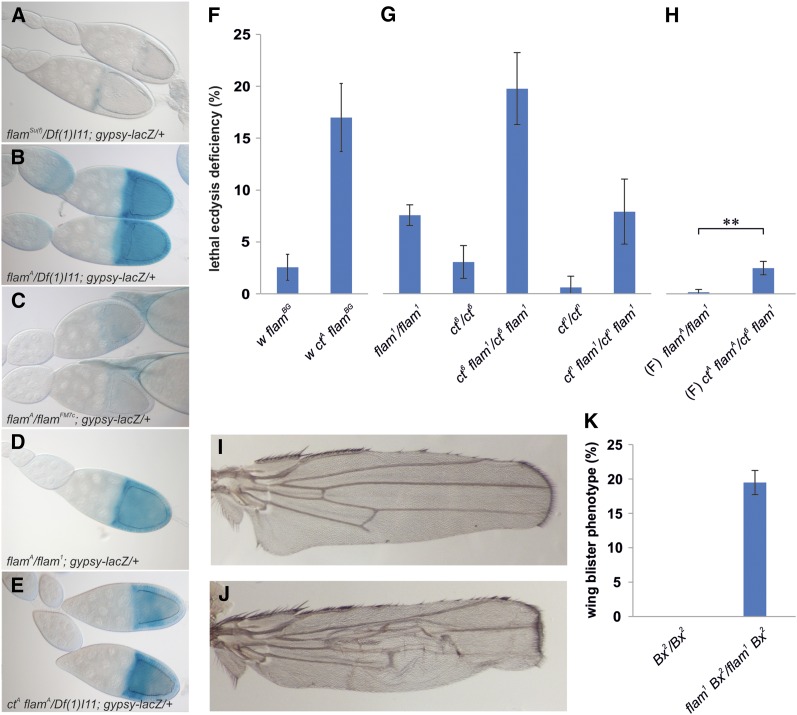
Figure 2.
Genetic interactions between flamenco permissive alleles and gypsy insertions induce specific phenotypes. (A–E) Representative pictures of egg chambers subjected to β-galactosidase staining as readout for gypsy-lacZ reporter activity. (A) gypsy-lacZ reporter activity is repressed when the flamsu(f) restrictive allele is combined with the Df(1)l11 deficiency encompassing the flamenco locus. (B) When the same deficiency is combined with the X chromosome currying the flamA allele, gypsy-lacZ reporter activity is strongly induced. (C) gypsy-lacZ reporter activity is repressed when the flamFM7c restrictive allele is combined with the flamA allele. (D) gypsy-lacZ reporter activity is induced in flamA/flam1 ovaries. (E) Combination of the Df(1)l11 deficiency with the ctA allele in the flamA-containing chromosome significantly reduces β-galactosidase staining. (F) Mortality during adult eclosion of ctA flamBG flies compared to the flamBG strain. (G) Mortality during adult eclosion of ct6 and ctn flies in their original genetic background and in combination with flam1. (H) Mortality during adult eclosion of flamA/flam1 and ctA flamA/ct6 flam1 female (F) flies (** P < 0.01). (I, J) Wings derived from male flies hemizygous for Bx2 allele in (I) its original genetic background and (J) in flam1 background. (K) Frequency of wing-blister phenotype of Bx2 wings in their original genetic background and in combination with flam1.
Since lethal ecdysis deficiency has never been reported to be a phenotype induced by gypsy insertions in the cut locus (Jack 1985), we hypothesized that these phenotypes in w ctA flamA flies should depend on a genetic interaction between the gypsy insertion and the flamenco permissive allele. To test this hypothesis, the ctA chromosome was recombined with a chromosome carrying the permissive flamBG allele (Mevel-Ninio et al. 2007). Mortality during adult eclosion of w ctA flamBG flies was higher than in control w flamBG flies (Figure 2F), and very similar to that found in w ctA flamA flies (Figure 1F). We also tried to confirm these data using two other ct alleles and the permissive flam1 allele. To achieve this goal, the ct6 allele, which is also induced by a gypsy insertion (Tchurikov et al. 1989), was recombined with flam1. These recombinant flies (ct6 flam1) also showed an increased lethal ecdysis deficiency with respect to flam1 and ct6 flies (Figure 2G). In addition, we found that the heteroallelic combination ctA flamA/ct6 flam1 produced a low but significant increase of lethal ecdysis deficiency with respect to flamA/flam1, confirming the specificity of the genetic interaction (Figure 2H). Since roo-specific small RNAs are not produced by the flamenco locus (Yang et al. 2010), we explored whether the ctn allele, which is induced by insertion of a roo element (Tchurikov et al. 1989), genetically interacts with flam1. The ctn flam1 recombinant flies did not show an increased level of ecdysis failure with respect to flam1 and ctn flies (Figure 2G). This demonstrated that flamenco does not interact with a cut mutation induced by a TE that is not under its control, confirming that the ecdysis deficiency phenotype is independent from the cut loss of function. Finally, we tested whether a gypsy-induced mutation in a different gene was able to genetically interact with flamenco. To achieve this, a gypsy mutation in the Beadex (Bx) gene was recombined with flam1. We used the gain-of-function mutation Bx2, which is known to induce scalloped wing margins (Figure 2I) (Shoresh et al. 1998). We found that the Bx2 flam1 recombinant showed wing blistering in addition to the wing-margin phenotype (Figure 2, J and K), confirming that a gypsy insertion in a gene different from cut in combination with a flamenco permissive allele can produce a specific phenotype.
All these observations support the hypothesis that the phenotypes we observed were specifically induced by a genetic interaction between a gypsy element inserted in a euchromatic site and a flamenco permissive allele.
flamenco activity is modulated by gypsy insertions into distant euchromatic loci
Due to the increase of gypsy copy number, a new gypsy insertion in a flamenco permissive background should result in a higher level of gypsy expression. Surprisingly, gypsy expression appeared significantly reduced in all stages and tissues analyzed from ctA flamA homozygous flies (Figure 3, B–E). This suggested that the presence of the ctA insertion produced a less permissive background for gypsy, which was confirmed by the reduction in gypsy-lacZ reporter activity in ovaries of ctA flamA/Df(1)l11 females (Figure 2E) compared to control (Figure 2B). Besides repression of the gypsy reporter, we found a significant reduction in the expression of endogenous gypsy elements (Figure S3A). To confirm that the downregulation of gypsy expression is specifically dependent on flamenco permissive alleles, the heteroallelic combination flamA/flam1 was also tested. We found a gypsy downregulation both when the X chromosomes carried two different gypsy insertions in the same locus, and when the X chromosomes carried two different gypsy insertions in different loci (Figure S3B); confirming the specificity of the effect. Then, we explored whether the new gypsy insertion in the cut locus had consequences on the regulation of other retrotransposons regulated by flamenco. To this aim, the expression levels of ZAM and Idefix were analyzed in adult heads and ovaries. We found that the expression of these two retrotransposons was significantly decreased in heads while it appeared unaffected in ovaries (Figure 3, D and E). These data confirmed that a euchromatic insertion of gypsy could modify the expression of other retrotransposons regulated by flamenco, but that this regulation is tissue-dependent. It is possible that in ovaries, where the Piwi pathway is active, the regulatory mechanism induced by the new gypsy insertion takes place in a different way.
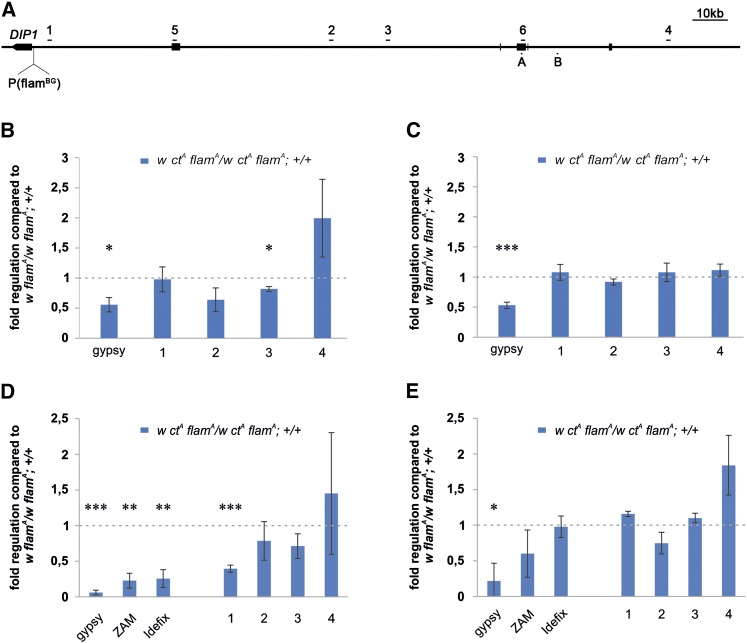
Figure 3.
The gypsy insertion in the cut locus induces changes in the expression of gypsy and flamenco. (A) Map of the flamenco cluster showing the DIP1 gene and the flamBG02658 (flamBG) insertion at the 5′ end of the locus, the gypsy transposon fragments (thick bars), the positions of primers used to analyze flamenco primary transcripts expression (numbers 1–6), and the position of tested piRNAs (letters A and B). (B–E) qRT-PCR analysis of gypsy, ZAM, Idefix, and specific flamenco regions in RNA isolated from (B) 48-hr-old pupae, (C) 96-hr-old pupae, (D) 0- to 24-hr-old female adult heads, and (E) 3-day-old adult ovaries. Shown are average levels (n = 3), and error bars indicate SD (* P < 0.05, ** P < 0.01, *** P < 0.005).
Since gypsy and other TEs regulated by flamenco showed a downregulation in the flamA permissive background, we hypothesized that the new gypsy insertion in the cut locus could also modify flamenco activity. Since flamenco is a source of small RNAs derived from the processing of a primary transcript, analysis of the expression levels at the flamenco locus could give an indication of the activity of the locus. Expression of the flamenco precursor transcript was analyzed by qRT-PCR using four pairs of primers designed to target different regions of the locus (Figure 3A). Two pairs were selected in sequences that map uniquely to the flamenco locus and are located at the 5′ end (primers 1) and in the central part (primers 3). The two other pairs of primers were specific for sequences that do not map uniquely to the flamenco locus (primers 2 and 4). The ctA mutation had a minor or no effect on the transcription of flamenco during pupal stages (Figure 3, B and C) or in ovary tissues (Figure 3E). However, in heads, we observed a significant reduction at the 5′ region of the flamenco locus (Figure 3D). These data are in agreement with the hypothesis that flamenco activity can be modified by a new gypsy insertion in a euchromatic region.
To confirm the causal effect of gypsy euchromatic insertions on the flamenco activity, we looked for novel spontaneous mutations in the original AstC-R2f03116 strain. We isolated a new mutation producing a forked-bristle phenotype (Figure S4A). This mutation, caused by a new gypsy insertion in the forked (f) gene, was named fA. When placed in in a y w67c23 background, the fA allele only produced a small increase in mortality during adult eclosion (Figure S4B), and induced significant changes in the expression levels of gypsy and of a sequence that does not map uniquely to the flamenco locus (Figure S4C). Differences in expression levels in regions mapping uniquely to the flamenco locus were also found (Figure 5B), confirming the causal effect of gypsy euchromatic insertions on the flamenco activity.
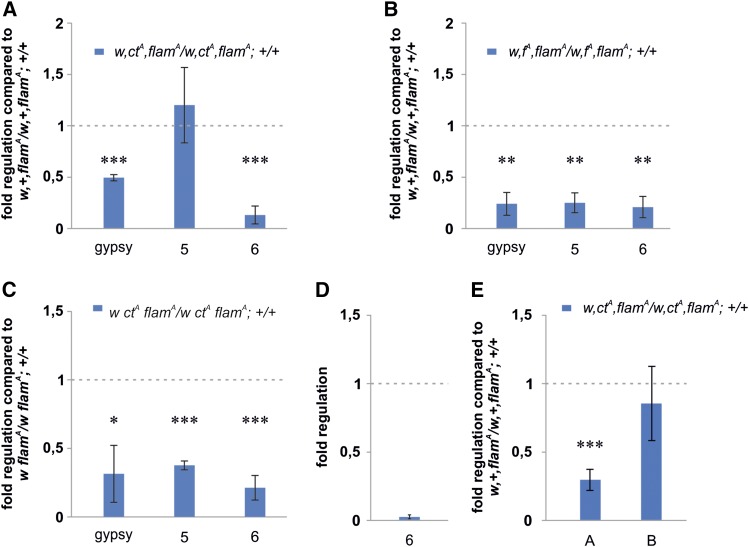
Figure 5.
Cosuppression of gypsy and flamenco sequences from which small RNAs against gypsy originate. qRT-PCR analysis. Shown are the transcript levels of gypsy and two gypsy fragments in the flamenco locus (primers 5–6, described in Figure 3A) in RNA isolated from 48-hr-old pupae carrying the homozygous (A) ctA or (B) fA mutation induced by gypsy. (C) Expression of gypsy and gypsy fragments inside flamenco in 0- to 24-hr-old adult heads from flies carrying the ctA homozygous mutation. (D) Expression of the distal gypsy fragment (primers 6) in w flamA adult ovaries compared to that in 48-hr-old pupae. (E) piRNA levels in 3-day-old adult ovary detected by TaqMan specific probes (see Materials and Methods for details). Position of analyzed piRNAs loci are indicated in Figure 3A. Shown are average levels (n = 3), and error bars indicate SD (* P < 0.05, ** P < 0.01, *** P < 0.005).
The ecdysis phenotype induced by the gypsy insertion in the cut locus is based on an RNA-silencing mechanism
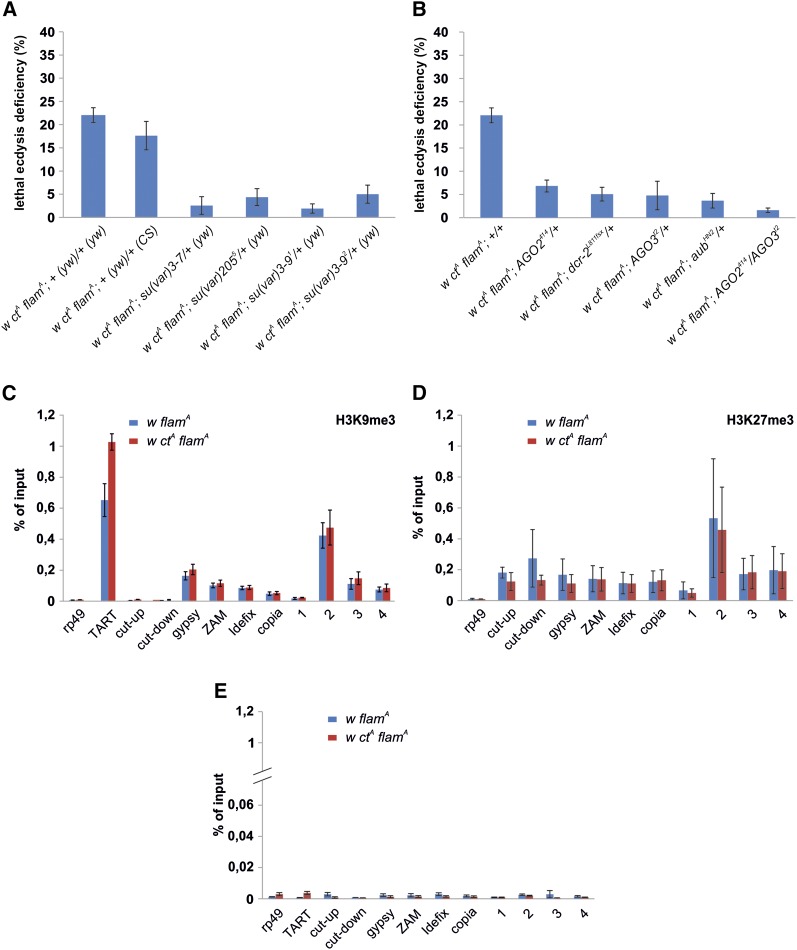
The involvement of an RNA-silencing mechanism is a possible hypothesis to explain in which way the gypsy insertion in the cut locus induces lethality during adult eclosion. Therefore we decided to analyze the effects of mutations affecting the production of endo-siRNAs and piRNAs on this lethal phenotype. However, since loss-of-function mutations of endo-siRNA and piRNA genes increase heterochromatin formation at flamenco and at other small RNA clusters (Moshkovich and Lei 2010), we wondered if mutations affecting heterochromatin formation per se could modify the adult ecdysis phenotype induced by ctA. Interestingly, we found that heterozygous mutations in Su(var)3-9, Su(var)205, and Su(var)3-7, which also suppress heterochromatic silencing at the flamenco locus (Moshkovich and Lei 2010), induced a strong reduction in the ecdysis phenotype (Figure 4A). Furthermore, we found that the increased lethal ecdysis deficiency found in ctA flamA carrying the TM6b balancer chromosome (Figure 1F) was associated with an enhancement of variegation both at the flamenco locus (Figure S5, A and B and File S1) and at pericentromeric loci (Figure S5C). All these data confirm that modifications in heterochromatin silencing at the flamenco locus affect the ecdysis phenotype induced by ctA. In addition, these observations suggested that analysis of homozygous mutants of the endo-siRNA and piRNA pathways should be avoided, since their effects on the heterochromatin of flamenco could mask any effects on post-transcriptional silencing. Notably, an increase of heterochromatin formation at the flamenco locus in AGO2 and piwi heterozygous mutants seems to be excluded by ChIP assays performed using α-HP1 antibodies (Moshkovich and Lei 2010). Furthermore, there are indications that some TEs are also activated in heterozygous RNA interference-mutant conditions (Xie et al. 2013), suggesting that a heterozygous condition can be sufficient to produce a partial TE activation. Based on these data, we decided to analyze the effects of mutations affecting endo-siRNAs and piRNAs in heterozygous conditions. We found that heterozygous AGO2414 and Dcr-2L811fsX mutations induced a significant reduction in lethal ecdysis phenotype (Figure 4B), supporting the hypothesis that the endo-siRNA pathway is involved in the post-transcriptional silencing of flamenco. Heterozygous mutations in aub and AGO3 genes, which are expressed in some somatic tissues (Specchia et al. 2008; Perrat et al. 2013), also produced a partial rescue of the lethal ecdysis deficiency induced by ctA (Figure 4B); indicating that piRNAs are also involved in the post-transcriptional silencing of flamenco. We also analyzed the effect of heterozygous mutations for both endo-siRNA and piRNA genes. The double heterozygous mutant produced an almost-complete rescue of the phenotype (Figure 4B), confirming the hypothesis that both these pathways are involved in the induction of the lethal ecdysis deficiency phenotype.
Figure 4.
Lethal ecdysis deficiency and flamenco modulation induced by gypsy seems to depend on post-transcriptional silencing. (A, B) Mortality during adult eclosion of ctA flamA flies carrying mutations affecting heterochromatin or small RNA pathways. (A) Analysis of mutations affecting heterochromatin formation. As an additional control, the heterozygous combination of a third chromosome from the yw67c23 strain and a third chromosome from the Canton-S strain was also analyzed. The genotype of the flies from which the third wild-type chromosome originates is enclosed in brackets. (B) Analysis of mutations affecting endo-siRNA and piRNA production. (C–E) ChIP analysis. Cross-linked chromatin of adult heads from w flamA and w ctA flamA females was IP with antibodies specific to (C) H3K9me3, (D) H3K27me3, and (E) no antibody as negative control. The IP DNA was analyzed by qPCR. Protein binding is expressed as the percentage of input and is shown for each primer set. cut-up and cut-down primers amplify upstream and downstream of the gypsy insertion at the cut locus, respectively. TART is used as a positive region for H3K9me3, and rp49 as a negative region showing background levels. Shown are average levels from two independent experiments and error bars indicate average deviation.
A possible explanation of the reduced flamenco expression in heads of flamA ctA flies was that the gypsy insertion in the cut locus could induce heterochromatic silencing at the flamenco locus. However, ChIP experiments did not reveal increases in levels of the heterochromatic marks H3K9me3 and H3K27me3 in flamA ctA heads (Figure 4, C–E), both in the analyzed regions of the flamenco locus and in the selected TE sequences. Although repressive marks were found from the gypsy sequence, we observed background levels of H3K9me3 and no increase in H3K27me3 into the genomic regions flanking the gypsy insertion (Figure 4, C–E, cut-up and cut-down primers). Based on these results, the observed changes in expression levels of gypsy and at the flamenco locus induced by ctA are not accompanied by heterochromatin changes, suggesting post-transcriptional silencing.
All these data suggest that the lethal ecdysis deficiency phenotype requires TE silencing pathways. The RNA-silencing mechanism inducing this phenotype seems to act at the post-transcriptional level but is sensitive to heterochromatin modifications.
Euchromatic insertions of gypsy modify the flamenco transcript processing
We found significant reductions in gypsy expression induced by euchromatic insertions of gypsy in all developmental stages and tissues analyzed (Figure 3 and Figure S3), and confirmed by the gypsy-lacZ reporter assay (Figure 2E). This downregulation occurs without an increase in heterochromatic marks (Figure 4, C–E), suggesting post-transcriptional silencing. Furthermore, silencing of gypsy is accompanied by alterations in expression of specific flamenco regions and of other TEs regulated by flamenco (Figure 3D). An attractive hypothesis was that the sequences complementary to gypsy in the flamenco locus, required to produce small RNAs against gypsy, were downregulated along with gypsy. To confirm this hypothesis, we explored whether gypsy insertions in euchromatic regions caused a decrease in gypsy fragment sequences inside the flamenco transcript. To this aim, we analyzed the expression levels of two separate gypsy fragments (see Figure 3A, primers 5 and 6) in w ctA flamA and w fA flamA 48-hr-old pupae, using specific primers that only amplify gypsy fragments inside flamenco. We observed a significant reduction in the expression of the gypsy fragments in w ctA flamA and in w fA flamA pupae (Figure 5, A and B). Similar results were observed when analyzing the expression pattern of the gypsy fragments in w ctA flamA adult heads (Figure 5C). Since the expression of the distal gypsy fragment was considerably low in the w flamA ovary (Figure 5D), we were unable to reliably test a reduction due to the ctA mutation by qPCR. Expression of gypsy fragments was also analyzed in 80EF and 42AB dual-strand piRNA clusters, both in adult heads and in ovaries, without finding significant changes (Figure S6, A and B). This suggests that flamenco has specific properties that make it a preferential target of the silencing promoted by a new euchromatic gypsy insertion.
It has been reported that a reduction in the expression of flamenco primary transcript is accompanied by a reduction in the levels of mature piRNAs originating from this sequence (Brennecke et al. 2007). Expression analysis by qRT-PCR of the level of piRNA A, which maps to the distal gypsy fragment at ∼850 bp upstream to the region amplified by primers 6 (Figure 3A, region A), revealed a significant reduction in ovaries (Figure 5E). A similar reduction was found in the somatic tissues of the head (Figure S7A). To confirm the reduction of piRNA A levels, the Piwi protein was IP from ovary extracts (Saito et al. 2006) and piRNAs from Piwi IP were subjected to northern blot analysis using a specific probe (Figure S7B and File S1). Although at a lower extent compared to the RT-qPCR analysis, this alternative experimental approach confirmed the downregulation of piRNAs mapping to region A in ctA flamA ovaries. Since ZAM expression is not significantly decreased in ctA flamA ovaries (Figure 3E), we analyzed the expression level of piRNA B originating from a ZAM fragment of flamenco (Figure 3A, region B) by qRT-PCR, without observing a significant reduction (Figure 5E). These data confirm that new gypsy euchromatic insertions promote a reduction in the expression of complementary regions in the flamenco transcript and of a piRNA originating from these sequences. The simplest explanation of the downregulation in the expression of gypsy fragment sequences inside the flamenco transcript and the reduction in specific piRNAs is a degradation of gypsy complementary sequences inside the flamenco primary transcript.
The gypsy insertion in the cut locus promotes production of fused transcripts containing gypsy and flanking genomic sequences
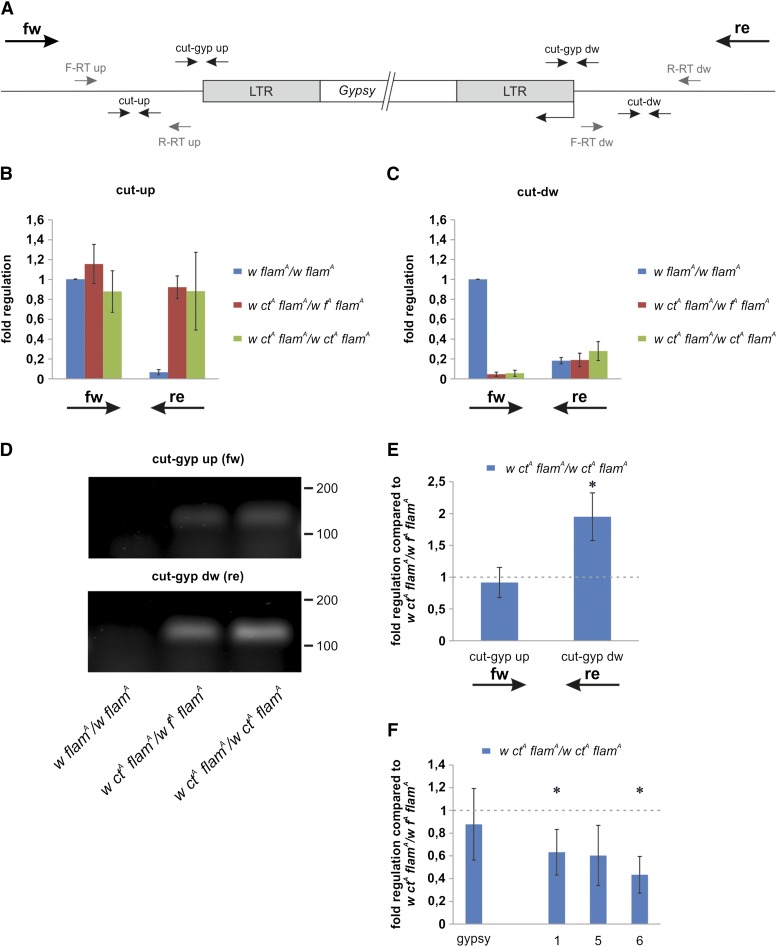
Decreased levels of gypsy and gypsy sequences in the flamenco transcript may be explained by a cosuppression triggered by the new gypsy insertion in the cut locus. This suggested that the gypsy insertion site becomes a new small RNA-generating locus able to silence gypsy sense and antisense sequences. This hypothesis would be supported by the fact that in the Drosophila germline, new euchromatic TE insertions induce a bidirectional transcription with formation of RNA containing TE and flanking genomic sequences, triggering the production of novel endo-siRNAs and pi-RNAs (Shpiz et al. 2014). We tested whether the gypsy insertion in the cut locus promotes the production of RNAs containing gypsy and flanking sequences by qPCR experiments performed using cDNAs obtained with strand-specific primers (Figure 6A). Expression in ctA flamA/ctA flamA and ctA flamA/fA flamA heads was compared with that in control heads (flamA/flamA). We used ctA flamA/fA flamA flies because they show a very low level of mortality during adult eclosion (Figure S4B), while they also carry two gypsy insertions as ctA flamA homozygous flies. This suggests that the effect on the flamenco locus is different in flies with a different pattern of gypsy insertions. We analyzed expression of the genomic region where the gypsy insertion of the ctA allele maps, finding a significant expression in the telomere to centromere (forward) direction and a very weak expression in the centromere to telomere (reverse) direction (Figure 6, B and C). Surprisingly, the presence of even a single ctA allele was sufficient to produce a strong activation in the reverse expression in the cut-up site and a strong suppression of the forward expression in the cut-down site (Figure 6, B and C). These data demonstrate that the gypsy insertions can modify expression of the regions surrounding the insertion site. We also found transcription from the flanking sequences into the gypsy sequence in the forward and reverse direction (Figure 6, D and E). Expression in the forward direction appeared quite similar, while the reverse expression of the ctA homozygous heads was about two times higher than that of ctA fA double heterozygous heads (Figure 6E). Reverse expression produces a fused transcript carrying sense gypsy sequences that can potentially be processed to produce small RNAs against gypsy antisense sequences. If these gypsy sense sequences served as a precursor for small RNAs, a lower level of gypsy sequences in the flamenco transcript from ctA homozygous heads compared to ctA fA double heterozygous heads would be expected. Indeed, expression of regions 5 and 6 was lower in ctA homozygous heads as well as expression of the 5′ of the flamenco transcript (Figure 6F). This suggests a higher level of degradation of the flamenco transcript in ctA homozygous heads. gypsy expression was not significantly different (Figure 6F), suggesting that the equal level of gypsy antisense sequences produces the same effect in ctA homozygotes and in ctA fA double heterozygotes. These data support the hypothesis that the gypsy insertion in the cut locus triggers the production of novel small RNAs directed against the sense sequence of the gypsy retrotransposon element and the gypsy antisense sequences that are part of the flamenco transcript.
Figure 6.
The gypsy insertion in the cut locus triggers the expression of fused transcripts from flanking genomic regions into the gypsy sequences. (A) Map of the gypsy insertion site in the cut locus and of the primers used in RT-PCR experiments (small arrows). Stand specific RT primers are represented in grey while qPCR primers are represented in black. fw arrow indicates direction of telomere-to-centromere expression while re arrow indicates direction of centromere-to-telomere expression. (B–F) RT-PCR experiments performed using RNA isolated from 0- to 24-hr-old female heads. (B, C) Strand-specific qRT-PCR reveals transcription in the genomic regions surrounding the gypsy insertion site in the cut locus. Transcription levels are variable and influenced by the pattern of the gypsy mutations present in each strain. Shown are average levels (n = 3), and error bars indicate SD. Retrotranscription of the cut-up site was primed by (B) primers F-RT up and R-RT up, while retrotranscription of the cut-down site was primed by (C) primers F-RT dw and R-RT dw. Primers cut-up were used to amplify the cut-up region while primers cut-dw were used to amplify the cut-down region. (D) Strand-specific RT-PCR products loaded in a 2% agarose gel confirming the presence of fused transcripts only in flies carrying the gypsy insertion in the cut locus. Primers cut-gyp up were used to amplify the forward fused transcript while primers cut-gyp dw were used to amplify the reverse fused transcript. DNA ladder scale is in base pairs. (E) Strand-specific qRT-PCR analysis of the transcription levels of the regions cut-gyp up and cut-gyp dw. Shown are average levels (n = 3), and error bars indicate SD (* P < 0.01). (F) qRT-PCR analysis. Shown are the transcript levels of gypsy and of three regions inside the flamenco locus (primers 1, 5, and 6; described in Figure 3A). Shown are average levels (n = 3), and error bars indicate SD (* P < 0.05).
Discussion
RNA-silencing mechanisms based on the production of small noncoding RNAs deriving from small RNA clusters have evolved to protect cells against TEs. Our understanding of the function of these clusters in the repression of TE activity is also derived from studies on the D. melanogaster flamenco locus. In the present work we found that new euchromatic gypsy insertions modify the activity of the flamenco locus and induce developmental alterations. This suggests that flamenco directly or indirectly regulates other processes in addition to TE silencing, and in fact it has been shown previously that insertional mutations in the flamenco locus induce alterations in oogenesis (Mevel-Ninio et al. 2007).
Phenotypes induced by euchromatic gypsy insertions seem to be very sensitive to the genetic background. In fact, we found significant changes in the frequency of the ecdysis deficiency phenotype induced by gypsy in presence of mutations or a balancer chromosome inducing epigenetic modifications. The low level of lethal ecdysis deficiency found in the heteroallelic combination ctA flamA/ct6 flam1 (Figure 2H) could be linked to the hybrid vigor, which also seems to depend on epigenetic changes that are found in hybrids (Chen 2013). Since mortality during adult eclosion induced by the gypsy insertion in the cut locus is modified by epigenetic modifications, it is likely that the weaker hybrid phenotypes have epigenetic causes. The number and localization of preexisting and new gypsy insertions in the different genetic backgrounds could also contribute to explain the variability (type and frequency) of the particular phenotypes arising from the genetic interaction between gypsy and flamenco. Regarding the localization of the gypsy insertions, we found different levels of lethal ecdysis deficiency in flies with the same genetic background, the same number of new gypsy mutations, and different gypsy localizations (Figure 1F and Figure S4B). Different effects were also found when the expression of gypsy and gypsy fragments inside the flamenco transcript were compared between two of these strains (Figure 6F).
The involvement of the piRNA pathway in the induction of the lethal ecdysis phenotype produced by ctA would confirm an important role of piRNA in somatic tissues. A contribution of maternal inheritance of piRNAs cannot be excluded, since it has been reported that epigenetic information can be transmitted to the offspring in the form of small RNAs (Akkouche et al. 2013). A deeper genetic analysis will be required to better understand how piRNAs operate using this mechanism.
A cosupression mechanism seems to be at the base of the altered flamenco activity induced by gypsy
We examined the effect of a new mutation induced by a gypsy insertion in the cut locus, which resulted in significant changes in the expression of specific sequences of the flamenco locus. We found that the expression of gypsy is downregulated as well as the expression of sequences that map uniquely to the flamenco locus inside regions that give rise to small RNAs which specifically target gypsy. This mechanism is reminiscent of the phenomenon described as cosuppression in plants, where a transgene reduces the expression of homologous sequences through a RNA-mediated silencing mechanism (Smyth 1997; Montgomery and Fire 1998). Overall, our data suggest that gypsy elements may cause post-transcriptional silencing of flamenco. This does not seem strange because small RNAs antisense to the flamenco transcript are found in databases (Yang et al. 2010), suggesting that small RNA clusters are natural targets of RNA silencing processes. Small RNAs antisense to flamenco also map to gypsy fragment sequences. It is known that endo-siRNAs and piRNAs also derive from TE sequences (Saito and Siomi 2010), and that in the Drosophila germline new euchromatic TE insertions trigger the production of endo-siRNAs and piRNas from TE and surrounding sequences (Shpiz et al. 2014). This suggests that the gypsy insertion in the cut locus may become a new small RNA-generating locus. Production of sense- and antisense-fused transcripts containing gypsy and the flanking genomic sequences at the gypsy insertion site in the cut locus (Figure 6, D and E) is in agreement with this hypothesis. Generation of new small RNAs from these gypsy sequences can explain the concomitant silencing of the gypsy retrotransposon and antisense gypsy fragment sequences. This can also explain the reduction in gypsy piRNA A level that we observed in ovaries and in adult heads. Reduction of piRNAs also exclude that the decreased levels of gypsy transcript and of flamenco precursor transcript derives from an increased production of flamenco piRNAs.
We did not find significant alterations in the expression of gypsy sequences located in other small RNA clusters (Figure S6). This may depend on the fact that 80EF and 42AB are dual-stranded clusters while flamenco is a single-stranded cluster, and they may have a different regulation. Expression of other gypsy fragments in different uni-strand and dual-strand clusters should be tested to confirm this hypothesis. Finally, we found that the region amplified by primers 2, which is a repeated sequence, may undergo significant changes (Figure S4C); suggesting that other genomic regions are involved in this regulatory mechanism. To have a broader view, it will be interesting to test if other TEs can modulate the activity of the small RNA clusters responsible for their suppression.
Reciprocal regulation of gypsy and flamenco probably influences the regulation of distal genes and sequences
Different experimental evidence indicates that flamenco, like other small RNAs clusters, is a heterochromatic region (Moshkovich and Lei 2010). Heterochromatin modifications can modulate flamenco activity, changing the expression pattern of the locus. However, we did not observe major changes in H3K9me3 and H3K27me3 enrichment at the flamenco locus in ctA homozygous flies (Figure 4, C–E). Furthermore, we did not find a spreading of heterochromatin in genomic sequences surrounding the gypsy insertion at the cut locus, as occurs when gypsy or other TEs are transcriptionally silenced by heterochromatic formation (Sienski et al. 2012). These findings suggest that the silencing of gypsy and flamenco that we found in the flamA background depends on a post-transcriptional mechanism. Since flamenco seems to be transcribed as a continuous single-stranded precursor spanning the locus (Brennecke et al. 2007; Goriaux et al. 2014a), differences in expression levels of specific flamenco regions are likely the result of differences in the processing of the precursor. In fact, differential changes in expression of specific regions of the flamenco locus have also been found in zucchini mutants, which have a role in the primary processing of piRNAs from the flamenco locus (Haase et al. 2010) and in comparing flamenco restrictive and permissive strains (Lavrenov et al. 2014). We propose that the differences in expression levels found at the specific flamenco regions analyzed, which are induced by new euchromatic gypsy insertions, depend on a degradation of specific sequences of the flamenco precursor transcript.
It is plausible that the high complexity of the small RNA clusters resulted in the acquisition of novel regulatory functions besides the original TE silencing function. A quantitative change in the amount of small RNAs, derived from flamenco and other small RNA clusters, can have an effect on the regulation of multiple target sequences. Potential targets regulated by flamenco are mRNAs that share sequence complementarity with small RNAs originating from flamenco and genes near the insertion site of gypsy, or of other TEs regulated by the small RNA cluster. It has been demonstrated that some TE-derived piRNAs and endo-siRNAs function as regulators of host genes (Rouget et al. 2010; McCue et al. 2012), and it has been suggested that TE-derived small noncoding RNAs may have a large influence on the transcriptome (McCue and Slotkin 2012). It is also known that when TEs are inserted into euchromatic regions, the expression of neighboring host genes can be modulated by ectopic recruitment of silencing factors (Slotkin and Martienssen 2007; Cowley and Oakey 2013). Although we did not find modifications in heterochromatic marks near to the gypsy insertion site into the cut locus, we cannot exclude that heterochromatin modifications can occur in other places near to other gypsy or TE sequences. This is supported by the fact that flamenco permissive alleles induce a genome-wide redistribution of the HP1 protein, as well as suppression of heterochromatic silencing in trans (Moshkovich and Lei 2010). We observed about a onefold increase in H3K9me3 level at the telomere-associated retrotransposon TART (Figure 4C), suggesting possible changes in heterochromatin distribution.
Identification of the target genes responsible for the different phenotypes induced by the gypsy insertions will be important to understand the mechanism by which the reciprocal regulation of gypsy and flamenco modifies fundamental developmental processes. This will help to clarify whether the developmental phenotypes induced by euchromatic gypsy insertions depend on the regulatory effects on the flamenco activity, or whether they directly depend on the production of new small RNAs from the gypsy insertion site. In the latter case, the lack of repression of gypsy due to the flamenco permissive background would favor the activation of the new small RNA cluster.
Acknowledgments
A special thanks to the late Franco Graziani, without whom this work would never have been possible. We thank Valeria Cavaliere, Giuseppe Gargiulo, Adriana La Volpe, Paolo Bazzicalupo, Umberto di Porzio, Aurora Storlazzi, and Massimo Di Giulio for helpful discussion, advice, and critical reading of the manuscript. We thank Silvia Andone and Yukiko Murota for technical support, Tamas Schauer for advice about chromatin preparation and immunoprecipitation, Marilena Ignesti for her useful advice, and Giovanna Lattanzi for her help during the revision of the manuscript. We thank the Institute of Genetics and Biophysics for use of the Integrated Microscopy Facility. We also thank Alain Pélisson for fly strains. This work was supported by the Italian Ministry of Economy and Finance “Medical Research Italy” (MERIT-RBNE08LN4P-002), the Italian Ministry of Economy and Finance grant project FaReBio di Qualità, the 2012 “5 per mille” project to the Rizzoli Laboratory of Musculoskeletal Cell Biology, and Deutsche Forschungsgemeinschaft (SFB 1064, SPP1356).
Footnotes
Communicating editor: J. A. Birchler
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.187922/-/DC1.
Literature Cited
- Akkouche A., Grentzinger T., Fablet M., Armenise C., Burlet N., et al. , 2013. Maternally deposited germline piRNAs silence the tirant retrotransposon in somatic cells. EMBO Rep. 14: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C. M., Quesneville H., Anxolabehere D., Ashburner M., 2006. Recurrent insertion and duplication generate networks of transposable element sequences in the Drosophila melanogaster genome. Genome Biol. 7: R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Aravin A. A., Stark A., Dus M., Kellis M., et al. , 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103. [DOI] [PubMed] [Google Scholar]
- Bucheton A., 1995. The relationship between the flamenco gene and gypsy in Drosophila: how to tame a retrovirus. Trends Genet. 11: 349–353. [DOI] [PubMed] [Google Scholar]
- Buchon N., Vaury C., 2006. RNAi: a defensive RNA-silencing against viruses and transposable elements. Heredity 96: 195–202. [DOI] [PubMed] [Google Scholar]
- Chalvet F., Teysset L., Terzian C., Prud’homme N., Santamaria P., et al. , 1999. Proviral amplification of the Gypsy endogenous retrovirus of Drosophila melanogaster involves env-independent invasion of the female germline. EMBO J. 18: 2659–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. J., 2013. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 14: 471–482. [DOI] [PubMed] [Google Scholar]
- Cowley M., Oakey R. J., 2013. Transposable elements re-wire and fine-tune the transcriptome. PLoS Genet. 9: e1003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B., Malone C. D., Zhou R., Stark A., Schlingeheyde C., et al. , 2008. An endogenous small interfering RNA pathway in Drosophila. Nature 453: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning A. P., Gu W., Castoe T. A., Batzer M. A., Pollock D. D., 2011. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 7: e1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desset S., Conte C., Dimitri P., Calco V., Dastugue B., et al. , 1999. Mobilization of two retroelements, ZAM and Idefix, in a novel unstable line of Drosophila melanogaster. Mol. Biol. Evol. 16: 54–66. [DOI] [PubMed] [Google Scholar]
- Desset S., Meignin C., Dastugue B., Vaury C., 2003. COM, a heterochromatic locus governing the control of independent endogenous retroviruses from Drosophila melanogaster. Genetics 164: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbary R. A., Lucas B. A., Maquat L. E., 2016. Retrotransposons as regulators of gene expression. Science 351: aac7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V., 2012. Presidential address. Transposable elements, epigenetics, and genome evolution. Science 338: 758–767. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M., Seitz H., Horwich M. D., Li C. J., Du T. T., et al. , 2008. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 320: 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriaux C., Desset S., Renaud Y., Vaury C., Brasset E., 2014a Transcriptional properties and splicing of the flamenco piRNA cluster. EMBO Rep. 15: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriaux C., Theron E., Brasset E., Vaury C., 2014b History of the discovery of a master locus producing piRNAs: the flamenco/COM locus in Drosophila melanogaster. Front. Genet. 5: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane L. S., Saito K., Nishida K. M., Miyoshi K., Kawamura Y., et al. , 2007. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315: 1587–1590. [DOI] [PubMed] [Google Scholar]
- Haase A. D., Fenoglio S., Muerdter F., Guzzardo P. M., Czech B., et al. , 2010. Probing the initiation and effector phases of the somatic piRNA pathway in Drosophila. Genes Dev. 24: 2499–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. A., Gdula D. A., Coyne R. S., Corces V. G., 1993. A Leucine-Zipper domain of the suppressor of hairy-wing protein mediates its repressive effect on enhancer function. Genes Dev. 7: 1966–1978. [DOI] [PubMed] [Google Scholar]
- Jack J. W., 1985. Molecular organization of the cut locus of Drosophila melanogaster. Cell 42: 869–876. [DOI] [PubMed] [Google Scholar]
- Kalmykova A. I., Klenov M. S., Gvozdev V. A., 2005. Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline. Nucleic Acids Res. 33: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrenov A. R., Nefedova L. N., Romanova N. I., Kim A. I., 2014. Expression of hp1 family genes and their plausible role in formation of flamenco phenotype in D. melanogaster. Biochemistry (Mosc.) 79: 1267–1272. [DOI] [PubMed] [Google Scholar]
- Li C., Vagin V. V., Lee S., Xu J., Ma S., et al. , 2009. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C. D., Brennecke J., Dus M., Stark A., Mccombie W. R., et al. , 2009. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 137: 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue A. D., Slotkin R. K., 2012. Transposable element small RNAs as regulators of gene expression. Trends Genet. 28: 616–623. [DOI] [PubMed] [Google Scholar]
- McCue A. D., Nuthikattu S., Reeder S. H., Slotkin R. K., 2012. Gene expression and stress response mediated by the epigenetic regulation of a transposable element small RNA. PLoS Genet. 8: e1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel-Ninio M., Pélisson A., Kinder J., Campos A. R., Bucheton A., 2007. The flamenco locus controls the gypsy and ZAM retroviruses and is required for Drosophila oogenesis. Genetics 175: 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita P., Boeke J. D., 2016. How retrotransposons shape genome regulation. Curr. Opin. Genet. Dev. 37: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery M. K., Fire A., 1998. Double-stranded RNA as a mediator in sequence-specific genetic silencing and co-suppression. Trends Genet. 14: 255–258. [DOI] [PubMed] [Google Scholar]
- Moshkovich N., Lei E. P., 2010. HP1 recruitment in the absence of argonaute proteins in Drosophila. PLoS Genet. 6: e1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pélisson A., Song S. U., Prudhomme N., Smith P. A., Bucheton A., et al. , 1994. Gypsy transposition correlates with the production of a retroviral envelope-like protein under the tissue-specific control of the Drosophila flamenco gene. EMBO J. 13: 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pélisson A., Teysset L., Chalvet F., Kim A., Prud’homme N., et al. , 1997. About the origin of retroviruses and the co-evolution of the gypsy retrovirus with the Drosophila flamenco host gene. Genetica 100: 29–37. [PubMed] [Google Scholar]
- Perrat P. N., Dasgupta S., Wang J., Theurkauf W., Weng Z. P., et al. , 2013. Transposition-driven genomic heterogeneity in the Drosophila brain. Science 340: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme N., Gans M., Masson M., Terzian C., Bucheton A., 1995. Flamenco, a gene controlling the gypsy retrovirus of Drosophila melanogaster. Genetics 139: 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget C., Papin C., Boureux A., Meunier A. C., Franco B., et al. , 2010. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467: 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Siomi M. C., 2010. Small RNA-mediated quiescence of transposable elements in animals. Dev. Cell 19: 687–697. [DOI] [PubMed] [Google Scholar]
- Saito K., Nishida K. M., Mori T., Kawamura Y., Miyoshi K., et al. , 2006. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 20: 2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarot E., Payen-Groschene G., Bucheton A., Pélisson A., 2004. Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics 166: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer T., Schwalie P. C., Handley A., Margulies C. E., Flicek P., et al. , 2013. CAST-ChIP maps cell-type-specific chromatin states in the Drosophila central nervous system. Cell Rep. 5: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoresh M., Orgad S., Shmueli O., Werczberger R., Gelbaum D., et al. , 1998. Overexpression Beadex mutations and loss-of-function heldup-a mutations in Drosophila affect the 3′ regulatory and coding components, respectively, of the Dlmo gene. Genetics 150: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpiz S., Ryazansky S., Olovnikov I., Abramov Y., Kalmykova A., 2014. Euchromatic transposon insertions trigger production of novel Pi- and endo-siRNAs at the target sites in the drosophila germline. PLoS Genet. 10: e1004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienski G., Donertas D., Brennecke J., 2012. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell 151: 964–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin R. K., Martienssen R., 2007. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 8: 272–285. [DOI] [PubMed] [Google Scholar]
- Smyth D. R., 1997. Gene silencing: cosuppression at a distance. Curr. Biol. 7: R793–R795. [DOI] [PubMed] [Google Scholar]
- Song S. U., Kurkulos M., Boeke J. D., Corces V. G., 1997. Infection of the germ line by retroviral particles produced in the follicle cells: a possible mechanism for the mobilization of the gypsy retroelement of Drosophila. Development 124: 2789–2798. [DOI] [PubMed] [Google Scholar]
- Specchia V., Benna C., Mazzotta G. M., Piccin A., Zordan M. A., et al. , 2008. Aubergine gene overexpression in somatic tissues of aubergine(sting) mutants interferes with the RNAi pathway of a yellow hairpin dsRNA in Drosophila melanogaster. Genetics 178: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchurikov N. A., Gerasimova T. I., Johnson T. K., Barbakar N. I., Kenzior A. L., et al. , 1989. Mobile elements and transposition events in the cut locus of Drosophila melanogaster. Mol. Gen. Genet. 219: 241–248. [DOI] [PubMed] [Google Scholar]
- Warren I. A., Naville M., Chalopin D., Levin P., Berger C. S., et al. , 2015. Evolutionary impact of transposable elements on genomic diversity and lineage-specific innovation in vertebrates. Chromosome Res. 23: 505–531. [DOI] [PubMed] [Google Scholar]
- Xie W., Donohue R. C., Birchler J. A., 2013. Quantitatively increased somatic transposition of transposable elements in Drosophila strains compromised for RNAi. PLoS One 8: e72163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. H., Shao P., Zhou H., Chen Y. Q., Qu L. H., 2010. DeepBase: a database for deeply annotating and mining deep sequencing data. Nucleic Acids Res. 38: D123–D130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni V., Eymery A., Coiffet M., Zytnicki M., Luyten I., et al. , 2013. Distribution, evolution, and diversity of retrotransposons at the flamenco locus reflect the regulatory properties of piRNA clusters. Proc. Natl. Acad. Sci. USA 110: 19842–19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Drosophila strains are available upon request.