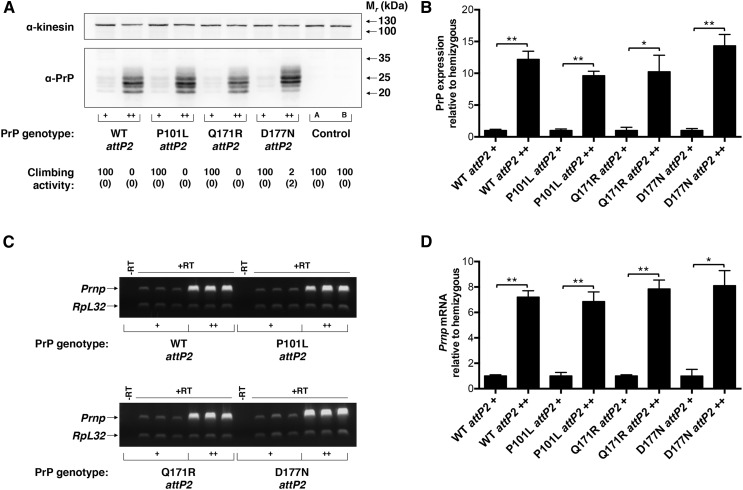
Figure 1.
Homozygosity of UAS-PrP at attP2 increases Prnp transcriptional efficacy. Four different UAS-PrP constructs, representing wild-type, pathogenic (P101L and D177N), and protective (Q171R) PrP sequences were inserted at attP2 and driven by Cha-GAL4. (A) Western blot of fly head homogenates from flies hemi- and homozygous for UAS-PrP constructs at attP2 (+, hemizygous; ++, homozygous). Experiment was performed in triplicate with a representative blot shown. Climbing activity of the various lines is shown below the corresponding lane of the Western blot and expressed as percent passing the climbing assay (see Materials and Methods) with standard deviation shown below in brackets. Control A is driver alone; control B is UAS-PrP alone. (B) Densitometry of Western blot signals in (A) and associated replicates. PrP signals were normalized to the kinesin loading control and then to the respective hemizygous UAS-PrP attP2 lines. (C) Semiquantitative RT-PCR performed on fly head mRNA of hemi- and homozygous attP2 UAS-PrP constructs driven by Cha-GAL4 (+, hemizygous; ++, homozygous). Prnp and RpL32 transcripts were coamplified from total cDNA, and each lane represents a biological replicate. Control samples, labeled –RT, represent PCR reactions of input RNA without addition of RT or oligo dT primer during the cDNA synthesis step. (D) Densitometry of RT-PCR signals in (C). For each sample, the ratio of Prnp to RpL32 mRNA levels was taken and then normalized to the average value for the respective hemizygous UAS-PrP attP2 line. Error bars in (B) and (D) represent standard deviations, with n = 3 for each group; ns denotes not significant, * P < 0.05, and ** P < 0.01, two-tailed Student’s t-test. cDNA, complementary DNA; mRNA, messenger RNA; PrP, prion protein; RT, reverse transcriptase; RT-PCR, reverse transcription polymerase chain reaction; WT, wild-type.