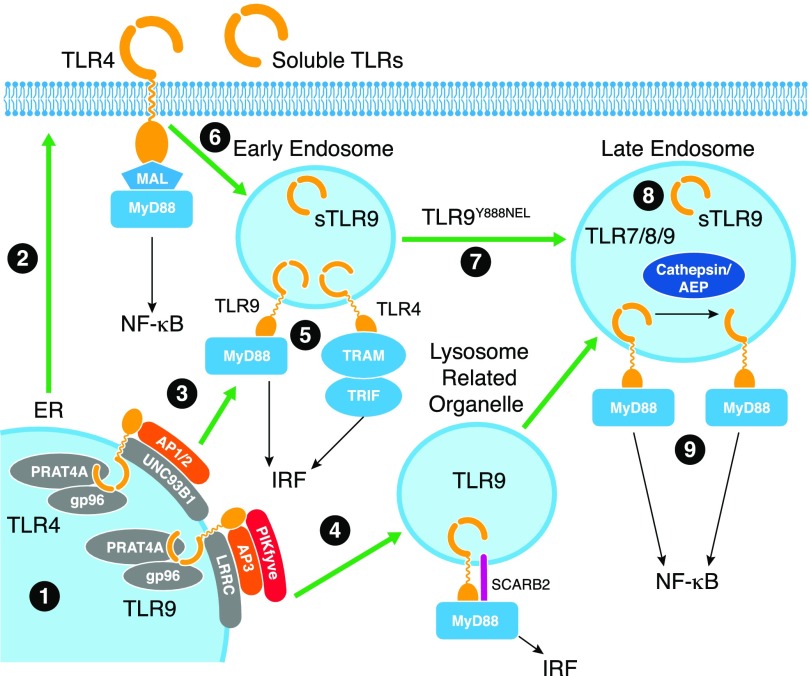
Figure 1. Regulation of TLR folding, trafficking, and cleavage.
1) TLRs are synthesized in the ER, and their correct folding requires gp96 and PRAT4A chaperones. 2) Surface TLRs follow traditional secretory pathways to access the cell surface. 3) Nucleic acid-sensing TLRs associate with UNC93B1, which itself associates with LRRC, and assists in trafficking to the early endosome. 4) Trafficking to endosomes in DCs depends on SCARB2, AP3, and PIKfyve to access the lysosome-related organelle. 5) Encounter with ligand in early endosomes or the lysosome-related organelle will elicit a signaling pathway, resulting in type I IFN production. In the early endosome, sTLR9 is generated that negatively regulates TLR9 signaling. 6) Surface TLR4 initiates signaling to the NF-κB pathway at the surface and upon internalization into the early endosome, reassembles a TRAM/TRIF complex on the TIR domain to elicit type I IFN production. 7) TLR9 trafficking from the early endosome to the lysosome depends on tyrosine 888 within a YNEL motif (single amino acids code). 8) Upon entry into this compartment, TLR9 and other nucleic acid-sensing TLRs are proteolytically cleaved by cathepsins and AEP to generate a mature form. 9) It is in this compartment that signaling leads to activation of the NF-κB pathway.