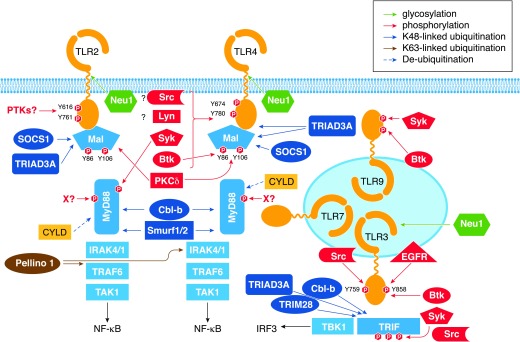
Figure 2. Control of TLR signaling by post-translational modifications.
Assembly of TLR complexes with adapters and kinases and formation of downstream signaling modules are regulated by multiple post-translational modifications. These include glycosylation of TLR2, TLR4, and TLR3 by Neu1; tyrosine phosphorylation of TLR2, TLR3, TLR4, TLR9, MyD88, and Mal by several kinases, including specific involvement of Src, Btk, Syk, Lyn, and EGFR (see text); and serine/threonine phosphorylation events regulating activity of TRIF and IRAK kinases. Several E3 Ub ligases regulate signaling via attachment of K63-linked polyubiquitination moieties to the respective substrates, such as IRAK1 and TBK1 (Pellino-1), promoting protein–protein interactions and downstream signaling or by conjugating K48-linked polyubiquitination residues to TLRs, Mal, MyD88, and TRIF (TRIAD3A, SOCS1, Smurf1/2, Cbl-b), targeting them for proteasomal degradation. CYLD deubiquitinating enzymes removes Ub residues from protein substrates, further fine-tuning TLR signaling.