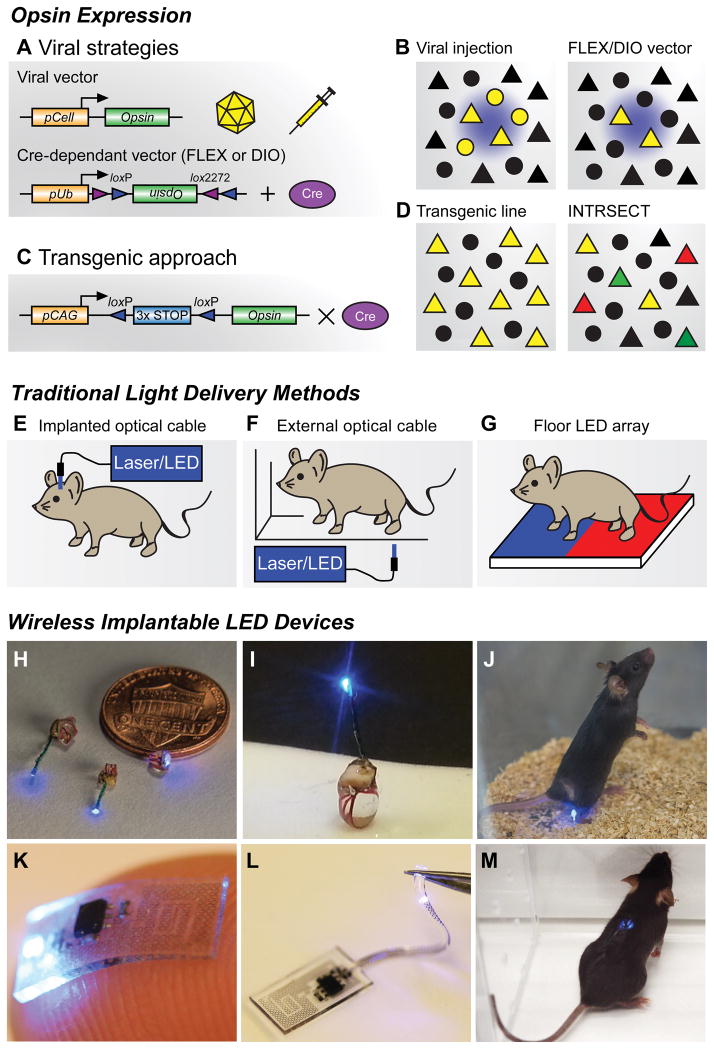
Figure 2. Opsin expression strategies and light delivery approaches.
(A) Viral vector strategies for delivering opsins in vivo. Transgene delivery most commonly uses injections of adeno-associated viral (AAV) vectors, though Herpes simplex virus and lentivirus can also be used. Specificity can be achieved by using a promoter of interest to restrict expression to specific cell types (pCell, top), however this approach can lead to weak or non-specific opsin expression. Cre-recombinase dependent vectors using flip-excision (FLEX) or double inverted orientation (DIO) targeting approaches (bottom). An inverted opsin gene (inactive) is flanked with two non-homologous recombination sites (loxP and lox2272). In the presence of Cre recombinase, the opsin is excised and flipped into a functional orientation. Because specificity is generated through tissue-specific expression of Cre-recombinase, strong ubiquitous promoters (pUb) can be used; however, these viruses must be injected in Cre-expressing transgenic animals (Cre database - http://www.gensat.org/cre.jsp).
(B) Cartoon illustrating focal transgene expression (yellow) from a non-Cre-dependent construct in a population of cells near the injection site (left). Similar example illustrating viral delivery of a FLEX/DIO vector into a transgenic animal with Cre-recombinase, depicted here with triangles (right).
(C) Transgenic approach for expressing opsins in vivo. Opsin constructs can be introduced into genetic loci to generate transgenic animals for conditional expression. In this approach, opsin genes are preceded by multiple stop codons to prevent expression. These stop codons are flanked by loxP sites, which are excised in the presence of Cre recombinase, generating strong cell-type and region-specific expression.
(D) Crossing transgenic opsin lines with Cre driver mice can grant optogenetic access to large neuronal populations (left). Further genetic refinement can be achieved using intersectional genetic approaches like INTRSECT (right). Here cellular specificity is controlled by using multiple recombination events with different enzymes. One group of genetically-defined cells is depicted in green, while a separate population is red. Yellow triangles represent the targeted subpopulation of cells that exhibit genetic features of both of groups.
(E) Different brain regions can be anatomically targeted by implanting optical fibers into cannulas affixed to the skull. Light is frequently delivered through a laser or LED light source; however, mice must be tethered to optical cables during behavioral experiments.
(F) Optical fibers can be used to illuminate external tissues with light from a laser or LED light source. While animals are untethered, access to deeper tissues is not possible, and uniform light delivery can be problematic.
(G) Opsins expressed in peripheral tissues of the paw can be stimulated by LED arrays placed in the floor of behavioral chambers, allowing for untethered movement during experiments. This approach can easily incorporate different stimulation wavelengths.
(H,I) Wireless implantable LED devices for stimulating superficial areas in the brain, spinal cord, and peripheral tissues. Reproduced from reference [68] with permission.
(J) Image of a freely moving animal with a LED device implanted in the hindpaw. Wireless activation is achieved via radio frequency waves generated by a resonant cavity below the chamber. This couples electromagnetic energy to the mouse, which is harvested by the implant. Reproduced from reference [68] with permission.
(K,L) Flexible wireless μLED devices designed to directly interface with the sciatic nerve (K), or be threaded into the epidural space of the spinal column to spinal cord (L) for optogenetic stimulation. Reproduced from reference [122] with permission.
(M) Image of a freely moving mouse with a flexible device implanted in the spinal column. Wireless activation is achieved by an external radio frequency antenna that directly powers the μLED device. Reproduced from reference [122] with permission.