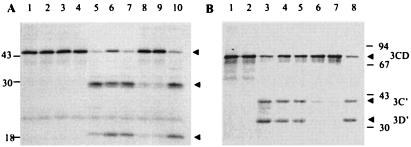
FIG. 3.
2BC inhibits 3Cpro activity. (A) In vitro-translated, [35S]methionine-labeled CREB was incubated with buffer (lane 1) or 500 ng each of bacterially expressed, affinity-purified 2B (lanes 2 and 6), 3Dpol (lanes 3 and 7), 2BC (lanes 4 and 9), and 2C (lane 8) in the absence (lanes 1 to 4) or presence (lanes 5 to 9) of 500 ng of 3Cpro. Lane 10 is the same as lane 5 except that 500 ng of an extract from bacteria harboring the plasmid without the 2C insert was used. (B) 2Apro activity is inhibited by 2C. Various amounts of 2C were preincubated with 20 ng of purified 2Apro before addition to the reactions containing [35S]methionine-labeled 3CD. Reactions were terminated by the addition of 2x SDS sample buffer and proteins resolved on a 14% SDS polyacrylamide gel. All reactions contain labeled [35S] methionine-labeled 3CD. Lane 1, control reaction with labeled 3CD as substrate plus buffer; lane 2, 500 ng of 2C; lane 3, 20 ng of 2Apro; lanes 4 to 7, reactions containing 2Apro (20 ng) preincubated with 125 ng (lane 4), 250 ng (lane 5), 375 ng (lane 6), 500 ng (lane 7) of 2C, or 500 ng of heat-inactivated 2C (lane 8) before the addition of labeled 3CD substrate. The arrowheads indicate full-length 3CD and proteolyzed products 3C′ and 3D′, respectively. Positions of the migration of molecular markers are indicated on the right (in kilodaltons).