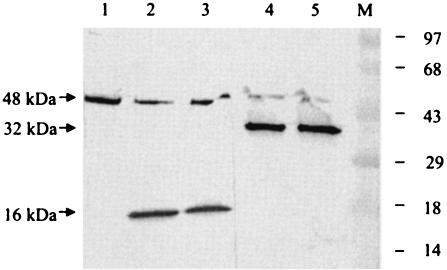
FIG. 4.
Effect of 2C protein on cellular proteases. Two eukaryotic proteases, thrombin, and enterokinase were used in this experiment. The activity of the proteases was monitored by Western blot analysis after cleavage of a control protein of 48 kDa supplied by the manufacturer (Novagen, Inc.) in the presence or absence of 2C polypeptide as detailed in Materials and Methods. Lane 1, control protein plus buffer; lane 2, control protein in the presence of 0.06 U of enterokinase; lane 3, same as lane 2 but also containing 500 ng of purified 2C; lane 4, control protein and 0.005 U of thrombin; lane 5, same as lane 4 but also containing 500 ng of purified 2C. The numbers on the right indicate the positions of the prestained molecular weight markers (lane M). The migrations of the 32- and 16-kDa cleaved products and the full-length protein are marked by arrows.