Abstract
Proteolytic cleavage of the Hendra virus fusion (F) protein results in the formation of disulfide-linked F1 and F2 subunits, with cleavage occurring after residue K109 in the sequence GDVK↓L. This unusual cleavage site and efficient propagation of Hendra virus in a furin-deficient cell line indicate that the Hendra F protein is not cleaved by furin, the protease responsible for proteolytic activation of many viral fusion proteins. To identify the subcellular site of Hendra F processing, Vero cells transfected with pCAGGS-Hendra F or pCAGGS-SV5 F were metabolically labeled and chased in the absence and presence of inhibitors of exocytosis. The addition of carbonyl-cyanide-3-chlorophenylhydrazone, monensin, brefeldin A, or NaF-AlCl3 or incubation of cells at 20°C all inhibited processing of the Hendra F protein, suggesting that cleavage of Hendra F occurs either in secretory vesicles budding from the trans-Golgi network or at the cell surface. In contrast to proteolytic cleavage of the simian virus 5 (SV5) F protein by the Ca2+-dependent protease furin, proteolytic cleavage of the Hendra F protein was not significantly inhibited by decreases in Ca2+ levels following incubation with EGTA or A23187. However, in the presence of weak amines and H+ V-ATPase inhibitors, known to raise intracellular pH, cleavage of Hendra F protein was inhibited while processing of the SV5 F protein was not significantly affected. The subcellular location, sensitivity to pH changes, and decreased Ca2+ requirement suggest that the protease responsible for cleavage of Hendra F protein differs from proteases previously shown to be involved in the processing of other viral glycoproteins.
A number of enveloped viruses, including paramyxoviruses and retroviruses, enter host cells via a pH-independent mechanism that facilitates fusion of the viral envelope with a membrane of the target cell. In paramyxoviruses, this process is mediated by the fusion (F) protein. The F protein is a type I integral homotrimeric, N-glycosylated membrane protein that is synthesized as an inactive precursor protein (F0). F0 is proteolytically cleaved to the disulfide-bonded F1 and F2 forms. This proteolytic event is critical for exposure of the fusion peptide and production of the mature and fusogenic form of the F protein.
Most paramyxovirus F proteins, including the simian virus 5 (SV5) F protein (20), the measles virus F protein (6), and the respiratory syncytial virus F protein (59), are proteolytically processed by furin. Furin is a secretory pathway protease that belongs to the family of mammalian subtilisin-like proprotein convertases (PCs). This membrane-associated, calcium (Ca2+)-dependent protease is known to process a wide range of precursor proteins within the exocytic and endocytic pathways at sites containing an Arg-X-Lys/Arg-Arg cleavage motif. Furin has additionally been described to proteolytically cleave a number of other viral glycoproteins, including the Ebola virus glycoprotein (Gp) (65), human immunodeficiency virus (HIV) gp160 (1, 28), the hemagglutinin (HA) protein of avian influenza virus strains H5 and H7 (58), and the cytomegalovirus glycoprotein B (63). Furthermore, a correlation of the efficiency of furin cleavage with virulence and pathogenicity has been described for both Newcastle disease virus (21) and Ebola virus (65). In contrast to intracellular proteolytic processing at a multibasic cleavage site by furin, a number of viral fusion proteins, such as Sendai virus F protein (31, 55) and H1, H2, and H3 subtypes of influenza virus HA protein (35), are cleaved at single basic residues by extracellular exogenous proteases. Cleavage of viral glycoproteins by proteases belonging to the pyrolysin branch of subtilisins has also been described (5, 37-39, 64). The Ca2+-dependent SKI-1/S1P protease was recently shown to proteolytically process the Lassa virus precursor protein (38, 39), the Crimean-Congo hemorrhagic fever virus glycoprotein (64), and the lymphocytic choriomeningitis virus glycoprotein (5, 37). SKI-1/S1P cleaves precursor proteins containing a conserved arginine at the fourth position and a hydrophobic amino acid at the second position prior to the cleavage motif and is predominantly active in the endoplasmic reticulum (ER), although the lymphocytic choriomeningitis virus glycoprotein is processed in a late Golgi compartment (5).
Hendra virus is a newly emerged zoonotic paramyxovirus that was first isolated in 1994 in Australia during an outbreak of severe respiratory illness that resulted in the deaths of 14 horses and 1 human (48). A second human fatality occurred 13 months later due to viral encephalitis as a result of an initial Hendra virus infection (53). Nipah virus, a virus closely related to Hendra virus, was isolated as the etiological agent of the fatal viral encephalitis outbreak that caused 105 fatalities out of 265 human cases in Malaysia in 1999. More than one million pigs were culled in order to contain the Nipah virus epidemic (11). Fruit bats are suspected to be the natural hosts for both Hendra and Nipah viruses (12, 19). The high rates of mortality in humans and the ability of Hendra and Nipah viruses to infect a number of different hosts have contributed to their classification as biosafety level 4 agents. Genomic and phylogenetic studies demonstrated high homology between Hendra and Nipah viruses, yet limited identity with other viruses within the Paramyxovirinae subfamily. The broad host range and antigenic cross-reactivity between Hendra and Nipah viruses, together with genomic features such as the large genome, unique genome terminal sequences, and a unique motif within the L protein, have supported the creation and classification of Hendra and Nipah viruses into a new genus within the Paramyxovirinae subfamily, namely, Henipaviruses (29, 67).
Hendra virus contains two glycoproteins, the attachment or G protein, which lacks both HA and neuraminidase activities, and the F protein. Similar to other paramyxoviruses, the Hendra virus F0 precursor protein is proteolytically cleaved into disulfide-linked subunits F1 and F2. The cleavage site (VGDVK109), predicted by amino acid sequence alignments, was confirmed by N-terminal sequencing of the F1 subunit (44). Cleavage of the closely related Nipah virus F protein similarly occurs after the basic residue arginine in the sequence VGDVR109 (28). Not only do the F proteins of both Hendra virus and Nipah virus lack the polybasic furin consensus motif, common to the majority of paramyxoviruses, but also the sequence at the site of proteolytic cleavage does not correspond to the recognition sequence of any known secretory protease. Successful growth of Hendra virus in the furin-deficient LoVo cell line confirmed that furin was not the protease involved in cleavage of the Hendra F protein (44). Furthermore, addition of exogenous trypsin did not affect propagation of Hendra virus in cell culture, indicating that an extracellular protease that cleaves at the basic residue is not required (44). In the present study, we have examined the subcellular location of cleavage as well as the Ca2+ and pH conditions required for efficient proteolytic processing of the Hendra F protein. We find that cleavage occurs either in the secretory vesicles budding from the trans-Golgi network (TGN) or at the cell surface. Cleavage is not significantly affected by decreases in cellular Ca2+ levels but is dramatically decreased by changes in intracellular pH. These differences suggest that the protease responsible for cleavage of the Hendra virus F protein differs from proteases previously shown to be involved in the processing of viral glycoproteins.
MATERIALS AND METHODS
Cell lines.
Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco Invitrogen, Carlsbad, Calif.). Human colon carcinoma LoVo cells were purchased from the American Type Culture Collection and maintained in Ham's F12 medium. Fetal bovine serum (FBS; 10%), penicillin, and streptomycin were added to all media.
Plasmid vectors.
Vectors containing the Hendra virus F and G genes were kindly provided by Lin-fa Wang (Australian Animal Health Laboratory). The F gene was released from the pUC18 vector by SalI digestion and ligated into XhoI-digested pCAGGS. Similarly, the G gene was excised from the pGEM-7T vector by NsiI and XhoI digestion and ligated into pCAGGS. Both F and G were cloned into pCAGGS in the correct orientation and sequenced to confirm that gene sequence integrity was maintained following subcloning. Robert Lamb (Howard Hughes Medical Institute [HHMI], Northwestern University) generously provided the pCAGGS-SV5 F and -SV5 HN expression vectors.
Antibodies.
Antipeptide antibodies (Genemed Custom Peptide Antibody Service, San Francisco, Calif.) were generated to amino acids 526 to 539 within the cytoplasmic tail of the Hendra F protein. SV5 F antipeptide antibodies to amino acids 82 to 96, within the SV5 F2 subunit, were kindly provided by Robert Lamb (HHMI, Northwestern University).
Expression of F and G (or HN) proteins.
F and G (or HN) proteins from Hendra virus and SV5 were transiently expressed by using the mammalian expression vector pCAGGS, which permits high levels of protein expression from a chicken actin promoter (49). Subconfluent monolayers in 35-mm tissue culture dishes were transiently transfected with plasmid DNA by using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Two micrograms of pCAGGS-Hendra F and/or 1 μg of pCAGGS-Hendra G, -SV5 F, or -SV5 HN, 6 μl of Plus reagent, and 4 μl of Lipofectamine in 0.8 ml of OPTI-MEM (Gibco Invitrogen) were combined and added to the monolayers. At 3 h posttransfection, cells were washed twice in phosphate-buffered saline and incubated overnight at 37°C in 2 ml of OPTI-MEM.
Pulse-chase experiments and immunoprecipitation.
Following transfection, pulse-chase experiments were performed. Cells were washed and starved for 45 min in DMEM deficient in methionine and cysteine. Tran35S-label (100 μCi/ml; MP Biomedicals, Inc., Irvine, Calif.) was added to the methionine- and cysteine-deficient DMEM, and cells were labeled for 30 min. The labeling medium was removed, cells were washed, and normal DMEM with or without 10% FBS (or 1% bovine serum albumin [BSA]) was added. Cells were then chased for various time intervals. At the end of the chase period, cells were washed twice with phosphate-buffered saline followed by lysis with RIPA buffer containing 100 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholic acid, protease inhibitors (1 KalliKrein inhibitory unit of aprotinin [Calbiochem, San Diego, Calif.], 1 mM phenylmethylsulfonyl fluoride [Sigma, St. Louis, Mo.], and Complete protease inhibitor tablets [Roche Molecular Biochemicals, Indianapolis, Ind.]) and 25 mM iodoacetamide (Sigma). The lysates were centrifuged at 136,500 × g for 10 min at 4°C, and supernatants were collected. Antipeptide sera and protein A-conjugated Sepharose beads (Amersham, Piscataway, N.J.) were used to immunoprecipitate the F proteins as previously described (54). Immunoprecipitated F proteins were analyzed via SDS-15% polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using the STORM imaging system (Amersham).
Inhibition of exocytic transport.
A number of different chemicals were used to inhibit exocytic transport within the cell. Monensin (20 μM; Sigma) and 5 μg of brefeldin A (Sigma)/ml were present throughout the pulse-chase experiment. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP; 50 μg/ml; Sigma) was added only to the chase medium. A mixture of 30 mM NaF-0.05 mM AlCl3 · 6H2O (Sigma) was included in the labeling and chase media. Inhibition of proteolytic cleavage was also examined by chasing F-transfected cells at 20 or 37°C for 2 h, followed by a further chase for 1 h at 37°C. Inhibitor assays used DMEM without FBS for the chase medium.
Cellular Ca2+ and pH manipulation assays.
Manipulation of intracellular Ca2+ concentrations was undertaken by including various concentrations of EGTA (Sigma) and A23187 (Calbiochem) in the label and chase media. For the Ca2+ assays, cells were starved and labeled in Ca2+-methionine-cysteine-deficient medium (Specialty Media, Phillipsburg, N.J.) and chased with minimal essential medium (Gibco Invitrogen).
Intracellular pH levels were modified by the addition of different concentrations of chloroquine (Sigma), NH4Cl (Sigma), bafilomycin A1 (Calbiochem), and concanamycin A (Calbiochem). Chloroquine and NH4Cl were present throughout the starvation, label, and chase periods, whereas bafilomycin A1 and concanamycin A were added only to the chase medium.
Endo H digestion.
Endoglycosidase H (Endo H) digestion of immunoprecipitated F proteins was performed as previously described (54). In brief, immunoprecipitated F proteins were boiled for 4 min in 0.4% SDS and 20 mM Na2HPO4 (pH 8). Supernatants were collected and incubated with 0.1 M sodium citrate (pH 5.3) and 1 mM phenylmethylsulfonyl fluoride in the absence or presence of 2 mU of Endo H (Roche Molecular Biochemicals) at 37°C for 27 h.
RESULTS
Proteolytic cleavage of the Hendra virus F protein.
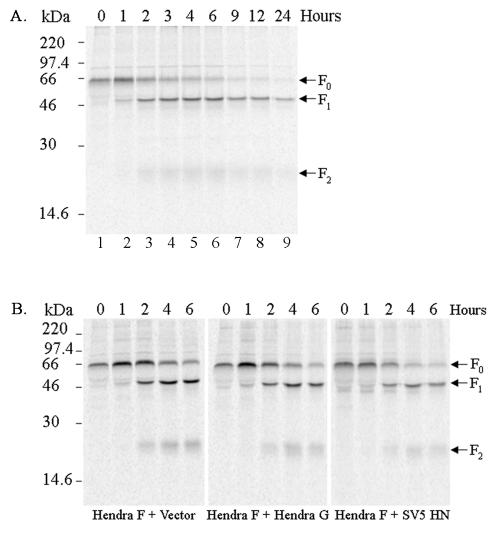
Synthesis and proteolytic cleavage of the Hendra virus F protein have been observed in several cell lines infected with the virus (44) as well as when the F protein was expressed using a recombinant vaccinia virus expression system (7). To examine the proteolytic processing of the Hendra virus F protein in the absence of viral infection, the Hendra F gene was subcloned into the mammalian expression vector pCAGGS (49). This vector allows for a high level of transient expression from the chicken actin promoter without the requirement of additional plasmid or virus. pCAGGS-Hendra F was transiently transfected into Vero cells, and proteolytic cleavage of the Hendra F protein was monitored by pulse-chase analysis (Fig. 1A). Cleavage of Hendra F protein was examined by immunoprecipitation of the F protein from lysed cells with a Hendra F-specific antibody to the cytoplasmic tail (526 to 539 amino acids), followed by separation on a 15% denaturing polyacrylamide gel and analysis on the STORM imaging system. The empty pCAGGS vector was transfected into Vero cells and used as a control. Immediately after labeling with Tran35S-label (0 h), only F0 the precursor form was apparent (Fig. 1A, lane 1). Cleavage of the Hendra F0 precursor to F1 and F2 subunits was observed following a 1-h chase, with levels of cleavage increasing during 1 to 4 h. Some F0 appeared to remain stable in the cell for long periods of time, as a small amount was still immunoprecipitated even after a 24-h chase (Fig. 1A, lane 9). As the analysis of proteolytic processing of the Hendra F protein was conducted over an extended time period (24 h), serum was included in the chase medium to maintain cell viability. Serum contains a plethora of proteins, including proteases and protease inhibitors. To exclude the possibility that serum proteins affected Hendra F0 cleavage, a truncated pulse-chase analysis (0 to 6 h) in the presence of DMEM and 1% BSA was undertaken. The extent of Hendra F0 processing and appearance of F1 and F2 with serum-free chase medium gave identical results to that observed in Fig. 1A (data not shown), suggesting that serum neither increases nor retards cleavage of the Hendra F protein. As the absence of FBS did not influence cleavage, subsequent experiments to determine the subcellular location and Ca2+ and pH requirements of the proteolytic event were performed in the absence of serum to avoid possible sequestration or degradation of the drugs. Additionally, as the majority of cleavage appears to occur within the first 4 h after synthesis and the F1 and F2 forms were clearly visible at 2 h after labeling, this time point was used in all subsequent inhibitor experiments. We also examined the expression and processing of the Hendra F protein by using the recombinant vaccinia virus-T7 RNA polymerase transient-expression system by pulse-chase analysis. Proteolytic cleavage of the Hendra F protein was not apparent until 6 h after labeling, with maximal cleavage observed at 12 h (data not shown), indicating that the pCAGGS expression system was a more appropriate system for study of proteolytic activation of the Hendra F protein.
FIG. 1.
Expression of the Hendra F protein. (A) Vero cells transfected with pCAGGS-Hendra F (2 μg) were starved for 45 min, labeled with 100 μCi of Tran35S-label/ml for 30 min, and either lysed immediately (0 h) or chased in DMEM with 10% FBS for 1, 2, 4, 6, 9, 12, and 24 h prior to lysis. Samples were immunoprecipitated, separated on an SDS-15% polyacrylamide gel, and analyzed by using the STORM imaging system. (B) Vero cells were cotransfected with pCAGGS-Hendra F (2 μg) and empty pCAGGS vector (1 μg); pCAGGS-Hendra F (2 μg) and pCAGGS-Hendra G (1 μg); or pCAGGS-Hendra F (2 μg) and pCAGGS-SV5 HN (1 μg). Transfected cells were metabolically labeled and chased in DMEM and 1% BSA for 0, 1, 2, 4, and 6 h. Samples were analyzed as before by immunoprecipitation, SDS-15% PAGE, and the STORM imaging system. The arrows on the right designate the positions of the F0, F1, and F2 proteins.
The presence of Hendra G, the attachment protein, is required for Hendra F-mediated membrane fusion (7). To determine whether Hendra G (the only other viral glycoprotein within the secretory pathway) enhanced proteolytic processing of the Hendra F protein, Hendra F and Hendra G were coexpressed in Vero cells and cleavage was examined over a 6-h time period. The empty pCAGGS vector as well as the SV5 attachment protein (HN) were cotransfected with Hendra F (in place of Hendra G) in parallel experiments as negative controls. The coexpression of either Hendra G or SV5 HN did not influence the cleavage of Hendra F or the amount of F protein processed (Fig. 1B), suggesting that the factor(s) needed for cleavage is present in uninfected Vero cells.
Furin does not proteolytically process the Hendra F protein.
An aberrant nucleotide deletion within the P domain of the furin gene in LoVo cells renders furin functionally inactive (60). The Hendra virus F protein lacks a furin cleavage motif, and infectious virus has been successfully propagated in LoVo cells (44). In order to compare the expression of Hendra F in furin-positive and -negative cell lines, pCAGGS-Hendra F was transfected into LoVo cells and cleavage was monitored over a 24-h time course by pulse-chase analysis. Similar to proteolytic processing of Hendra F in Vero cells, precursor F0 was observed immediately following metabolic labeling (0 h) (Fig. 2, lane 1), and cleavage to the F1 and F2 disulfide-linked forms similarly occurred during the chase period (Fig. 2, lanes 2 to 7). Overall expression levels in LoVo cells were decreased, making detection of the F2 subunit more difficult. The similar kinetics of proteolytic processing of the Hendra F protein in this cell line confirmed that furin is not involved in proteolytically cleaving Hendra F.
FIG. 2.
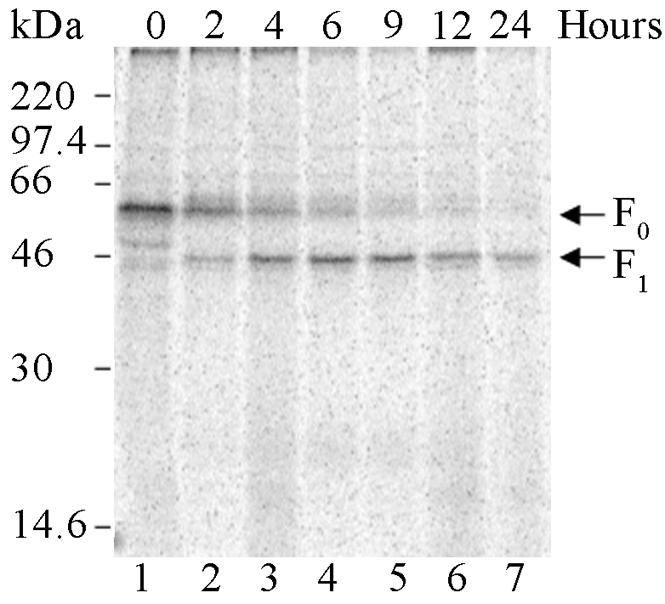
The Hendra F protein is proteolytically processed in LoVo cells. LoVo cells were transiently transfected with pCAGGS-Hendra F (2 μg), metabolically labeled, and lysed immediately (0 h) or chased in DMEM and 10% FBS for various times prior to lysis. Samples were immunoprecipitated and analyzed via SDS-15% PAGE and by the STORM imaging system. The F0 and F1 proteins are marked with arrows on the right.
Inhibitors of exocytic transport abolish proteolytic processing of the Hendra F protein.
Infectious Hendra virus has been successfully propagated in different cell lines in the absence of exogenous trypsin (44, 48), indicating that an extracellular protease that cleaves at the basic residue is not required. Additionally, it has been established that the Hendra F protein is not proteolytically processed by the secretory protease furin. To determine the subcellular location of Hendra F protein processing, pCAGGS-Hendra F-transfected Vero cells were metabolically labeled and incubated in the absence or presence of compounds known to affect exocytic transport. To examine whether proteolytic processing of the Hendra F protein occurs within the ER, an uncoupler of oxidative phosphorylation, CCCP, which is known to block the transport of proteins from the ER (15), was included in the chase medium. Cleavage of the Hendra F0 protein to generate F1 and F2 subunits was not inhibited in the presence of dimethyl sulfoxide alone (Fig. 3, lane 2); however, proteolytic processing of the Hendra F protein was completely blocked following incubation of 50 μg of CCCP/ml in dimethyl sulfoxide (Fig. 3, lane 3). These results demonstrate that cleavage of the Hendra F protein does not occur in the ER.
FIG. 3.
Inhibitors of vesicular transport block proteolytic processing of the Hendra F protein. Vero cells transfected with pCAGGS-Hendra F (2 μg) were starved for 45 min, labeled with Tran35S-label, and immediately lysed or chased for 2 h in DMEM in the absence (−) or presence (+) of 50 μg of CCCP/ml, 20 μM monensin, and 5 μg of brefeldin A/ml. Monensin and brefeldin A were included in the starvation, label, and chase media, while CCCP was present only in the chase medium. Samples were immunoprecipitated, separated on an SDS-15% polyacrylamide gel, and analyzed by use of the STORM imaging system. The uncleaved and cleaved forms of the Hendra F protein are designated with arrows on the right.
Vesicular transport between the medial and trans-Golgi cisternae is inhibited by the sodium ionophore monensin (26). Therefore, to determine whether proteolytic processing of the Hendra F protein occurred in the early compartments of the Golgi stack, pCAGGS-Hendra F-transfected Vero cells were metabolically labeled and chased for 2 h in the absence or presence of 20 μM monensin. Monensin was present throughout the pulse-chase experiment. Hendra F protein cleavage occurred in the presence of methanol (control) (Fig. 3, lane 4) but was completely abolished in the presence of monensin (in methanol) (Fig. 3, lane 5). Proteolytic cleavage of the Hendra F protein was also monitored by Endo H digestion. Endo H specifically digests high-mannose carbohydrates. In the medial Golgi, modifications and additions to high-mannose carbohydrates produce complex carbohydrates refractory to digestion by Endo H (54). Therefore, Endo H processing is often used as a marker of vesicular transport. Vero cells transfected with pCAGGS-Hendra F were metabolically labeled and chased for 0, 0.5, 1, 2, and 6 h. Endo H digestion of immunoprecipitated Hendra F protein showed that F0 became Endo H resistant prior to the cleavage event (data not shown). The inhibition of cleavage in the presence of monensin as well as Endo H resistance prior to cleavage suggest that protease activity resides in a compartment distal to the medial Golgi.
The fungal metabolite brefeldin A prevents anterograde trafficking from the ER while maintaining retrograde transport from the cis-, medial, and trans-Golgi (17, 40). The effect of brefeldin A on Hendra F cleavage was examined by the incorporation of brefeldin A in methanol (5 μg/ml) into the starvation, label, and chase media of pCAGGS-Hendra F-transfected Vero cells. In the presence of brefeldin A, Hendra F protein proteolytic processing was prevented (Fig. 3, lane 7). These data suggest that either redistributed enzymes, as a result of the Golgi stack collapse, were nonfunctional within the ER environment or that cleavage of the Hendra F protein occurs in the TGN or at the cellular surface. As the presence of each of these vesicular transport inhibitors abolished proteolytic cleavage of the Hendra F protein, processing occurs in the TGN or at the cell surface.
Similar to many paramyxoviruses, the SV5 F protein contains a polybasic cleavage motif and is known to be processed by furin (20) in the TGN. As a control, the effects of each of these inhibitors on proteolytic cleavage of the SV5 F protein were studied. CCCP, monensin, and brefeldin A similarly disrupted cleavage of the SV5 F protein, consistent with cleavage in the TGN (data not shown).
Cleavage of the Hendra F protein occurs either in the secretory vesicles or at the cell surface.
The formation of secretory vesicles from the trans-Golgi has been shown to be inhibited by the treatment of cells with a mixture of NaF and AlCl3 (3). To analyze whether proteolytic processing of the Hendra F protein occurs before or after the formation of secretory vesicles in the TGN, Vero cells transiently expressing Hendra F or SV5 F (as a positive control) were metabolically labeled and chased for 2 h as described previously. The 30 mM NaF-0.05 mM AlCl3 mixture was included in the label and chase periods. Although the presence of NaF-AlCl3 did not affect expression of the Hendra and SV5 F0 protein, cleavage to the F1 and F2 forms was prevented (Fig. 4A, lanes 4 and 8). We noted with SV5 F that immediately after labeling with Tran35S-label, a small amount of cleavage had already occurred. Additionally, a second band migrating slightly ahead of F1 was visible, although this band was no longer visible following the 2-h chase in DMEM. This additional band may represent either a degraded form of the SV5 F product or a labeled cellular protein that is subsequently degraded during the 2-h chase.
FIG. 4.
Proteolytic processing of Hendra F occurs proximal to the trans-Golgi. (A) Vero cells were transfected with pCAGGS-Hendra F (2 μg) and -SV5 F (1 μg) expression plasmids, metabolically labeled with Tran35S-label, chased for 2 h in DMEM in the absence (−) or presence (+) of 30 mM NaF-0.05 mM AlCl3, and analyzed by SDS-15% PAGE and the STORM imaging system. The NaF-AlCl3 mixture was included in the labeling and chase media. (B) Vero cells were transfected with pCAGGS-Hendra F (2 μg) and -SV5 F (1 μg), metabolically labeled, and chased for 2 h at either 37 or 20°C, followed by an additional 1-h incubation at 37°C. Samples were immunoprecipitated and analyzed as described above. The arrows on the right designate the positions of the F0, F1, and F2 proteins.
Temperature block experiments have been useful in examining trafficking of secretory and membrane proteins. Incubation of cells at 20°C influences both exocytic (43) and endocytic transport (25). Budding of immature secretory vesicles from the TGN and transport of newly synthesized proteins to the plasma membrane are prevented when cells are incubated at 20°C (43). Therefore, a temperature shift assay was undertaken to confirm results from the NaF-AlCl3 experiment. Vero cells transfected with pCAGGS-Hendra F and SV5 F (as a control) were metabolically labeled as before. Following labeling, transfected cells were chased either at 20 or 37°C for 2 h. Cleaved Hendra and SV5 F products were clearly visible following the 2-h chase at 37°C (Fig. 4B, lanes 2 and 7). In contrast, proteolytic processing of both Hendra and SV5 F0 was blocked when cells were chased at 20°C (Fig. 4B, lanes 4 and 9). However, when cells were shifted back to 37°C for an additional hour, restoring budding and transport of secretory vesicles, cleavage was observed (Fig. 4B, lanes 5 and 10). Inhibition of SV5 F proteolysis at 20°C is consistent with cleavage by furin occurring in the secretory vesicles budding from the TGN. Thus, proteolytic processing of the Hendra F protein was inhibited by both NaF-AlCl3 and a 20°C temperature shift, indicating that cleavage occurred either in the secretory vesicles budding from the TGN or at the cell surface.
A decrease in intracellular Ca2+ levels does not significantly influence proteolytic processing of the Hendra F protein.
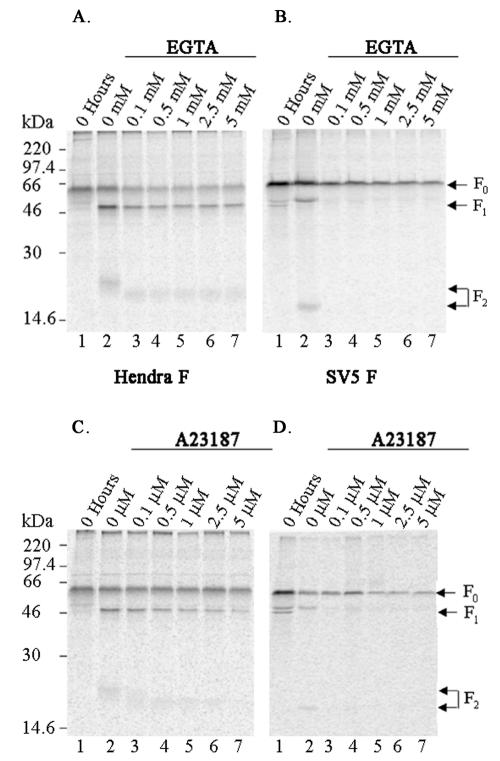
Changes in intracellular Ca2+ have been shown to influence the proteolytic activity of cellular secretory proteases, such as furin and other members of the PC family, including the ER protease SKI-1/S1P (56, 57). Therefore, the effect on cleavage of Hendra F following changes in intracellular Ca2+ levels was examined. pCAGGS-Hendra F-transfected and SV5 F-transfected (as a positive control) Vero cells were metabolically labeled, and Ca2+ levels were depleted by incubation during the label and chase periods with various concentrations of EGTA in Ca2+-deficient medium. Consistent with the Ca2+ dependence of furin, incubation with EGTA significantly reduced the processing of SV5 F, even at concentrations as low as 0.1 mM (Fig. 5B, lane 3). In contrast, the addition of EGTA up to 5 mM did not significantly affect cleavage of the Hendra F protein (Fig. 5A, lanes 3 to 7). A slight decrease in molecular weight of the Hendra F2 protein was also observed (Fig. 5A, lanes 3 to 7). Changes in intracellular Ca2+ levels may influence carbohydrate modifications (42) and, thus, may explain the molecular weight shift. Cleavage of the Hendra F protein was also analyzed in the presence of various concentrations of the Ca2+ ionophore A23187 (0.1 to 5 μM) (Fig. 5C). A23187 similarly reduced the amount of cleavage of the SV5 F protein (Fig. 5D, lanes 3 to 7) but did not significantly perturb proteolytic processing of the Hendra F protein (Fig. 5C, lanes 3 to 7). Cleavage of Hendra F was slightly reduced at the highest A23187 concentrations (Fig. 5C, lanes 6 and 7). This decrease in proteolytic processing may either be due to a toxic effect of A23187 at such concentrations or may indicate that the Hendra F protein cleavage event is not completely independent of Ca2+. Similar to experiments performed in the presence of EGTA, the molecular weight of the Hendra F2 subunit decreased following the addition of A23187 (Fig. 5C, lanes 3 to 7). Thus, manipulations of intracellular Ca2+ do not significantly alter proteolytic processing of the Hendra F protein, suggesting that the protease involved in the processing of Hendra F appears to have a decreased Ca2+ requirement compared to that of furin or SKI-1/S1P.
FIG. 5.
A decrease in calcium does not significantly affect proteolytic cleavage of the Hendra F protein. Vero cells were transfected with pCAGGS-Hendra F (2 μg; A and C) or -SV5 F (1 μg; B and D) expression plasmids and metabolically labeled in the absence (−) or presence (+) of increasing concentrations of EGTA (A and B) or A23187 (C and D). EGTA and A23187 were added to label and chase media, and calcium-free media were used throughout the experiment. Samples were immunoprecipitated and analyzed as described before. Precursor F0 and cleaved F1 and F2 subunits are indicated by the arrows on the right.
Proteolytic processing of Hendra virus F is inhibited by changes in intracellular pH.
The pH within subcompartments of the secretory pathway is thought to play an important role in their normal functioning (2). Proteins undergoing transport through the secretory pathway from the ER to TGN encounter pH changes from 7.1 to 5.91 (16, 36, 41). Our data suggest that the Hendra F protein is proteolytically processed either in the secretory vesicles budding from the TGN or at the cell surface, two locations with remarkably different pHs. It was therefore of interest to examine whether manipulations of intracellular pH affected cleavage of Hendra F. Weak basic amines such as chloroquine and NH4Cl (32, 50), as well as inhibitors of the vacuolar H+ V-ATPase, bafilomycin A1 and concanamycin A (18, 41), have been shown to raise intracellular pH. Vero cells transiently expressing Hendra F and SV5 F were metabolically labeled in the presence of increasing concentrations of chloroquine (1 to 100 μM), NH4Cl (1 to 20 mM), or bafilomycin A1 or concanamycin A (10 to 100 nM). Furin is known to be proteolytically active in the TGN and at the cell surface, as well as in endocytic vesicles, and has been characterized to be active over a broad pH range (pH 6 to 8) (62). The finding that increasing concentrations of chloroquine did not inhibit cleavage of the SV5 F protein was thus expected (Fig. 6B, lanes 3 to 6), with reduction only seen at 100 μM chloroquine (Fig. 6B, lane 7). At this concentration, chloroquine may have influenced initial synthesis and folding of SV5 F in the ER, reduced or abolished vesicular transport, or adversely affected the proteolytic activity of furin. Surprisingly, chloroquine concentrations greater than 1 μM abolished proteolytic processing of the Hendra F protein (Fig. 6A, lanes 3 to 7). As seen with SV5 F, the overall amount of Hendra F0 decreased in the presence of 100 μM chloroquine (Fig. 6A, lane 7), similarly suggesting that at this high concentration initial synthesis and folding of Hendra F in the ER may be retarded. NH4Cl (10 mM), bafilomycin A1 (50 nM), and concanamycin A (10 nM) similarly inhibited cleavage of Hendra F but not that of SV5 F (data not shown). Inhibition of Hendra F0 cleavage could potentially be due to the accumulation of F0 in either the ER or Golgi complex. To exclude this possibility, vesicular trafficking of Hendra F following pH manipulations was monitored by Endo H digestion. As can be seen in Fig. 6C (lanes 1 to 4), Endo H digestion of Hendra F from untreated cells immediately after labeling and the 2-h chase period permitted identification of Endo H-sensitive and -resistant F0 (Fos and For) and F1 (F1r) forms. Endo H-resistant Hendra F0 following treatment with 10 μM chloroquine, 50 nM bafilomycin A, and 10 nM concanamycin A (Fig. 6C, lanes 6, 10, and 12, respectively) was identified, suggesting that the Hendra F protein was efficiently transported through the medial Golgi. In contrast, treatment with 10 mM NH4Cl retarded exocytic transport, as a predominantly Endo H-sensitive form of Hendra F0 was identified (Fig. 6C, lane 8). Therefore, changes in intracellular pH inhibit proteolytic processing of Hendra F, and this effect is not due to a blockage of exocytic transport.
FIG. 6.
Changes in intracellular pH retard proteolytic processing of Hendra F but not the SV5 F protein. Vero cells were transfected with pCAGGS-Hendra F (2 μg; A) or -SV5 F (1 μg; B) expression plasmids, metabolically labeled, and chased for 2 h in DMEM. Increasing concentrations of chloroquine were present in the starvation, label, and chase media. Cells were lysed, immunoprecipitated, and analyzed by SDS-15% PAGE and the STORM imaging system. The arrows on the right designate the positions of the F0, F1, and F2 proteins. (C) pCAGGS-Hendra F-transfected Vero cells were metabolically labeled and chased as before in the absence (−) or presence (+) of 10 μM chloroquine, 10 mM NH4Cl, 50 nM bafilomycin A1, or 10 nM concanamycin A. Chloroquine and NH4Cl were present in the starvation, label, and chase media, and bafilomycin A1 and concanamycin A were present only in the chase medium. Samples were lysed, immunopreciptated, and either mock treated (−) or treated with 2 mU of Endo H (+) for 27 h at 37°C prior to analysis by SDS-10% PAGE and the STORM imaging system. Endo H-sensitive and -resistant F0 (F0s and For) and F1 (F1r) forms are indicated by the arrows on the right.
DISCUSSION
Hendra virus and Nipah virus are two newly emerged paramyxoviruses. The high mortality rates in humans and the ability of these viruses to infect a number of different hosts have contributed to their classification as biosafety level 4 agents. Although epidemiological and genomic studies have been undertaken, our understanding of cellular factors contributing to cell tropism, cell infection, viral transmission, and even host cell response to Hendra virus infection is in its infancy. The Hendra F protein contains several functional domains previously identified in paramyxoviruses and other type I viral fusion proteins. These include a signal peptide sequence, the fusion peptide, heptad repeat regions, and a transmembrane domain. However, the proteolytic cleavage of the Hendra F protein clearly differs from that seen with the F proteins of many paramyxoviruses. Instead of containing a polybasic cleavage motif that is processed by furin, cleavage of precursor F0 to the F1 and F2 subunits occurs after a single lysine residue (44). The use of diverse cell lines to analyze the propagation of infectious Hendra virus (44) and Hendra F processing for the promotion of fusion (7, 8) suggest that the protease is intracellularly active and ubiquitously expressed. To understand this processing event, we investigated the subcellular location and Ca2+ and pH requirements for proteolytic processing of the Hendra F protein.
When the Hendra F protein was expressed in Vero cells by using the mammalian expression plasmid pCAGGS, the precursor Hendra F0 protein was efficiently expressed and, like other paramyxovirus F proteins, proteolytically processed to the disulfide-linked forms, F1 and F2 (Fig. 1A). Although coexpression of the G protein is essential to promote Hendra and Nipah virus homotypic (and heterotypic) fusion (7, 8, 61), expression of Hendra G with Hendra F did not significantly affect cleavage efficiency (Fig. 1B), suggesting that other viral glycoproteins are not needed for processing and that cleavage is performed by a cellular protease. Additionally, the cleavage efficiency of Hendra F in the absence or presence of serum was examined. In contrast to the roles of serum plasminogen (23) and extracellular proteases (24) reported in the cleavage of influenza virus HA, the absence or presence of serum had no discernible effect on the processing of the Hendra F protein, suggesting that proteolytic activation of the Hendra F protein was not promoted by an extracellular protease. Furthermore, furin may be excluded as the intracellular processing protease, as the Hendra F protein was proteolytically cleaved in the furin-deficient LoVo cell line (Fig. 2).
Inhibitors of exocytosis have been used previously to define the subcellular location of proteolytic processing for respiratory syncytial virus F protein (13) and measles virus F protein (6). In our studies, inhibition of Hendra F cleavage in the presence of CCCP, monensin, and brefeldin A (Fig. 3) indicated that cleavage occurred either in the TGN or at the cell surface (Fig. 3). Temperature shift assays have been previously used to identify the subcellular location of proteolytic processing of the influenza virus HA and HIV gp160 proteins. Cleavage of influenza virus HA (43) and HIV gp160 (66) was mostly abolished following incubation at 20°C, which prevented vesicular trafficking by inhibiting the budding of immature secretory vesicles from the TGN. Processing of HIV gp160 was also inhibited following incubation of HIV gp160-transfected cells in the presence of the mixture of NaF-AlCl3 (65), known to prevent the formation of secretory vesicles from the TGN. Using these techniques (Fig. 4), we found that proteolytic maturation of the Hendra F protein was similarly inhibited, indicating that the Hendra F processing event occurred either in secretory vesicles budding from the TGN or at the cell surface.
Although cleavage of the Hendra F protein at the cell surface cannot be definitively excluded, the sensitivity of Hendra F processing to increases in intracellular pH (Fig. 6) strongly argues that the proteolytic event occurs intracellularly. Previous studies examining proteolytic processing of proalbumin (50) and precursor complement protein (proC3) (51) used weak basic amines such as chloroquine and NH4Cl to establish that maturation occurred in an acidic intracellular compartment, namely the Golgi complex, where furin is now recognized as the processing enzyme (45). Early virus entry studies used chloroquine to determine whether virus entry was a pH-dependent process or an independent process (9, 14). More recently, bafilomycin A1, a H+ V-ATPase inhibitor, has been employed to investigate the entry and fusion mechanisms of Semliki Forest virus and Sindbis virus (22), the role of the H+ V-ATPase in influenza virus entry (27), and the involvement of acidic organelles in herpes simplex virus assembly (30). Few studies, however, have examined the effect of intracellular pH on the folding, transport, and posttranslational modifications of viral glycoproteins. Initial biochemical studies of HIV gp160 maturation demonstrated that 20 mM NH4Cl affected intracellular transport and thus proteolytic maturation of HIV gp160 (68). In our studies, cleavage of the SV5 F protein was not significantly inhibited by the addition of weak basic amines or H+ V-ATPase inhibitors although, at high concentrations of chloroquine (100 μM) and NH4Cl (20 mM), the amount of SV5 F processing was reduced. However, our data clearly demonstrate the surprising result that chemically induced increases in pH have a dramatic inhibitory effect on the proteolytic processing of the Hendra F protein. Though concanamycin A has been shown to have an inhibitory effect on intracellular transport of the vesicular stomatitis virus glycoprotein G (47), the Endo H resistance of the Hendra F protein expressed in Vero cells with pH-modifying compounds demonstrates that vesicular trafficking of Hendra F past the medial Golgi was not affected by the presence of chloroquine, bafilomycin A1, or concanamycin A. Our results therefore demonstrate that the Hendra F proteolytic event is sensitive to increases in intracellular pH levels, suggesting that cleavage occurs in secretory vesicles budding from the TGN, and our results also indicate that the protease involved in this process is highly sensitive to changes in pH. Alternatively, the low-pH environment in the TGN may promote a conformational change in the Hendra F protein that facilitates proteolytic cleavage.
In addition, we have found that processing of the Hendra F protein is not affected by reductions of intracellular Ca2+ levels (Fig. 5). This decreased sensitivity to intracellular Ca2+ changes further suggests that the mechanism of proteolytic processing of the Hendra F protein differs from that previously described for viral glycoproteins. Although incubation in the presence of either EGTA or A23187 did not perturb cleavage of the Hendra F0 protein, the amount of proteolytic processing of the SV5 F protein was reduced, consistent with cleavage of SV5 F by the Ca2+-dependent furin. The measles virus F protein and HIV gp160 are similarly processed by furin, and the extent of fusion protein processing and syncytium formation of the measles virus F protein (6) and HIV gp160 (46) were similarly affected by A23187 concentrations above 0.125 μM. While an alternative proteolytic maturation of gp160 via a Ca2+-independent nonfurin mechanism has been proposed (4, 33, 34, 52), the putative proteases involved have yet to be cloned and identified, and their role in gp160 activation in vitro is yet to be confirmed. SKI-1/S1P has been shown to cleave a number of viral glycoproteins (5, 39, 64) but, like other subtilisin-like PCs, it is Ca2+ dependent. Both the Ca2+ independence and different cleavage site motif of the Hendra virus F protein suggest that SKI-1/S1P is not the protease responsible for cleaving the Hendra F protein.
To conclude, Hendra virus is a newly emerged zoonotic paramyxovirus. Similar to other paramyxoviruses, the Hendra virus fusion protein is proteolytically modified. However, the amino acids at the cleavage site and the efficient propagation of Hendra virus in furin-negative LoVo cells suggested that the Hendra F protein is uniquely processed. We have used chemical inhibitors to determine that cleavage of the Hendra F protein occurs either in secretory vesicles budding from the TGN or at the cell surface. The subcellular location, sensitivity to pH changes, and decreased Ca2+ requirement suggest that the protease responsible for processing the Hendra F protein differs from proteases previously shown to be involved in the maturation of viral glycoproteins. Our characterization of this process sets the stage for subsequent purification and identification of this critical cellular enzyme. Hendra virus and Nipah virus are potent pathogens for which no approved antiviral therapies currently exist. A 42-amino-acid peptide directed to the C-terminal heptad repeat region of the Hendra F protein has been demonstrated to inhibit in vitro F-mediated fusion (7). Additionally, a decrease in Nipah virus encephalitis mortality has been described following treatment with the antiviral ribavirin (10). As proteolytic cleavage of the fusion protein of paramyxoviruses is essential to the fusogenic activity of the F protein and, hence, virus infectivity and spread, targeting the protease responsible for this cleavage event may offer an alternative direction for antiviral therapy to both Hendra and Nipah viruses.
Acknowledgments
We thank Lin-fa Wang of the Australian Animal Health Laboratory for the Hendra F and G plasmids and Robert Lamb (HHMI, Northwestern University) for the pCAGGS-SV5 F and -HN expression vectors and SV5 F-specific antibodies. We are grateful to Carole Moncman and members of the Dutch lab for critically reviewing the manuscript.
C.T.P. is the recipient of a Research Challenge Trust Fund fellowship from the University of Kentucky. This study was supported by NIAID grant A151517 to R.E.D.
REFERENCES
- 1.Anderson, E. D., L. Thomas, J. S. Hayflick, and G. Thomas. 1993. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem. 268:24887-24891. [PubMed] [Google Scholar]
- 2.Anderson, R. G., and L. Orci. 1988. A view of acidic intracellular compartments. J. Cell Biol. 106:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr, F. A., A. Leyte, S. Mollner, T. Pfeuffer, S. A. Tooze, and W. Huttner. 1991. Trimeric G-proteins of the trans-Golgi network are involved in the formation of constitutive secretory vesicles and immature secretory granules. FEBS Lett. 294:239-243. [DOI] [PubMed] [Google Scholar]
- 4.Bendjennat, M., B. Bahbouhi, and E. Bahraoui. 2001. Purification and characterization of a Ca2+-independent endoprotease activity from peripheral blood lymphocytes: involvement in HIV-1 gp160 maturation. Biochemistry 40:4800-4810. [DOI] [PubMed] [Google Scholar]
- 5.Beyer, W. R., D. Pöpplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolt, G., and I. R. Pedersen. 1998. The role of subtilisin-like proprotein convertases for cleavage of the measles virus fusion glycoprotein in different cell types. Virology 252:387-398. [DOI] [PubMed] [Google Scholar]
- 7.Bossart, K. N., L.-F. Wang, B. Eaton, and C. C. Broder. 2001. Functional expression and membrane fusion tropism of the envelope glycoproteins of Hendra virus. Virology 290:121-135. [DOI] [PubMed] [Google Scholar]
- 8.Bossart, K. N., L.-F. Wang, M. N. Flora, K. B. Chua, S. K. Lam, B. T. Eaton, and C. C. Broder. 2002. Membrane fusion tropism and heterotypic functional activities of the Nipah virus and Hendra virus envelope glycoproteins. J. Virol. 76:11186-11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassell, S., J. Edwards, and D. T. Brown. 1984. Effects of lysosomotropic weak bases on infection of BHK-21 cells by Sindbis virus. J. Virol. 52:857-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong, H.-T., A. Kamarulzaman, C.-T. Tan, S. R. Kunjapan, N.-K. Chew, K.-B. Chua, and S.-K. Lam. 2001. Treatment of acute Nipah encephalitis with ribavirin. Ann. Neurol. 49:810-813. [DOI] [PubMed] [Google Scholar]
- 11.Chua, K. B., W. J. Bellini, P. A. Rota, B. H. Harcourt, A. Tamin, S. K. Lam, T. G. Ksiazek, P. E. Rollin, S. R. Zaki, W. Shieh, C. S. Goldsmith, D. J. Gubler, J. T. Roehrig, B. Eaton, A. R. Gould, J. Olson, H. Field, P. Daniels, A. E. Ling, C. J. Peters, L. J. Anderson, and B. W. Mahy. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432-1435. [DOI] [PubMed] [Google Scholar]
- 12.Chua, K. B., C. L. Koh, P. S. Hooi, K. F. Wee, J. H. Khong, B. H. Chua, Y. P. Chan, M. E. Lim, and S. K. Lam. 2002. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 4:145-151. [DOI] [PubMed] [Google Scholar]
- 13.Collins, P. L., and G. Mottet. 1991. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 72:3095-3101. [DOI] [PubMed] [Google Scholar]
- 14.Coombs, K., E. Mann, J. Edwards, and D. T. Brown. 1981. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J. Virol. 37:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copeland, C. S., K.-P. Zimmer, K. R. Wagner, G. A. Healey, I. Mellman, and A. Helenius. 1998. Folding, trimerization, and transport are sequential events in the biogenesis of Influenza virus hemagglutinin. Cell 53:197-209. [DOI] [PubMed] [Google Scholar]
- 16.Demaurex, N., W. Furuya, S. D'Souza, J. S. Bonifacino, and S. Grinstein. 1998. Mechanism of acidfication of the trans-Golgi Network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273:2044-2051. [DOI] [PubMed] [Google Scholar]
- 17.Doms, R. W., G. Russ, and J. W. Yewdell. 1989. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dröse, S., K. U. Bindseil, E. J. Bowman, A. Siebers, A. Zeeck, and K. Altendorf. 1993. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry 32:3902-3906. [DOI] [PubMed] [Google Scholar]
- 19.Field, H., P. Young, J. M. Yob, J. Mills, L. Hall, and J. Mackenzie. 2001. The natural history of Hendra and Nipah viruses. Microbes Infect. 3:307-314. [DOI] [PubMed] [Google Scholar]
- 20.Garten, W., S. Hallenberger, D. Ortmann, W. Schäfer, M. Vey, H. Angliker, E. Shaw, and H. D. Klenk. 1994. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie 76:217-225. [DOI] [PubMed] [Google Scholar]
- 21.Glickman, R. L., R. J. Syddall, R. M. Iorio, J. P. Sheehan, and M. A. Bratt. 1988. Quantitative basic residue requirements in the cleavage-activation site of the fusion glycoprotein as a determinant of virulence for Newcastle disease virus. J. Virol. 62:354-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 23.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 95:10224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotoh, B., T. Ogasawara, T. Toyoda, N. M. Inocencio, M. Hamaguchi, and Y. Nagai. 1990. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 9:4189-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths, G., S. Pfeiffer, K. Simons, and K. S. Matlin. 1985. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J. Cell Biol. 101:949-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths, G., P. Quinn, and G. Warren. 1983. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J. Cell Biol. 96:835-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallenberger, S., V. Bosch, H. Angliker, E. Shaw, H.-D. Klenk, and W. Garten. 1992. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360:358-361. [DOI] [PubMed] [Google Scholar]
- 29.Harcourt, B. H., A. Tamin, T. G. Ksiazek, P. E. Rollin, L. J. Anderson, W. J. Bellini, and P. A. Rota. 2000. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology 271:334-349. [DOI] [PubMed] [Google Scholar]
- 30.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homma, M. 1971. Trypsin action on the growth of Sendai virus in tissue culture cells. I. Restoration of the infectivity for L cells by direct action of trypsin on L cell-borne Sendai virus. J. Virol. 8:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hortin, G., and A. W. Strauss. 1986. Effects of acidotropic compounds on the secretory pathway: inhibition of secretion and processing of the third and fourth components of complement. Biochem. Biophys. Res. Commun. 136:603-609. [DOI] [PubMed] [Google Scholar]
- 33.Kamoshita, K., M. Shiota, M. Sasaki, Y. Koga, Y. Okumura, and H. Kido. 1995. Calcium requirement and inhibitor spectrum for intracellular HIV type I gp160 processing in cultured HeLa cells and CD4+ lymphocytes: similarity to those of viral envelope glycoprotein maturase. J. Biochem. 117:1244-1253. [DOI] [PubMed] [Google Scholar]
- 34.Kido, H., K. Kamoshita, A. Fukutomi, and N. Katunuma. 1993. Processing protease for gp160 human immunodeficiency virus type I envelope glycoprotein precursor in human T4+ lymphocytes. J. Biol. Chem. 268:13406-13413. [PubMed] [Google Scholar]
- 35.Kido, H., Y. Yokogoshi, K. Sakai, M. Tashiro, Y. Kishino, A. Fukutomi, and N. Katunuma. 1992. Isolation and characterization of a novel trypsin-like protease found in rat bronchiolar epithelial Clara cells. J. Biol. Chem. 267:13573-13579. [PubMed] [Google Scholar]
- 36.Kim, J. H., L. Johannes, B. Goud, C. Antony, C. A. Lingwood, R. Daneman, and S. Grinstein. 1998. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl. Acad. Sci. USA 95:2997-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunz, S., K. H. Edelmann, J.-C. de la Torre, R. Gorney, and M. B. A. Oldstone. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168-178. [DOI] [PubMed] [Google Scholar]
- 38.Lenz, O., J. ter Meulen, H. Feldmann, H.-D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenz, O., J. ter Meulen, H.-D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippincott-Schwartz, J., L. C. Yuan, J. S. Bonifacino, and R. D. Klausner. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llopis, J., J. M. McCaffery, A. Miyawaki, M. Farquhar, and R. Y. Tsien. 1998. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 95:6803-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodish, H. F., and N. Kong. 1990. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J. Biol. Chem. 265:10893-10899. [PubMed] [Google Scholar]
- 43.Matlin, K. S., and K. Simons. 1983. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34:233-243. [DOI] [PubMed] [Google Scholar]
- 44.Michalski, W. P., G. Crameri, L.-F. Wang, B. J. Shiell, and B. Eaton. 2000. The cleavage activation and sites of glycosylation in the fusion protein of Hendra virus. Virus Res. 69:83-93. [DOI] [PubMed] [Google Scholar]
- 45.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9:28-35. [DOI] [PubMed] [Google Scholar]
- 46.Moulard, M., L. Montagnier, and E. Bahraoui. 1994. Effects of calcium ions on proteolytic processing of HIV-1 gp160 precursor and cell fusion. FEBS Lett. 338:281-284. [DOI] [PubMed] [Google Scholar]
- 47.Muroi, M., A. Takasu, M. Yamasaki, and A. Takatsuki. 1993. Folimycin (concanamycin A), an inhibitor of V-type H+-ATPase, blocks cell-surface expression of virus envelope glycoproteins. Biochem. Biophys. Res. Commun. 193:999-1005. [DOI] [PubMed] [Google Scholar]
- 48.Murray, K., P. Selleck, P. Hooper, A. Hyatt, A. Gould, L. Gleeson, H. Westbury, L. Hiley, L. Selvey, B. Rodwell, and P. Ketterer. 1995. A morbillivirus that caused fatal disease in horses and humans. Science 268:94-97. [DOI] [PubMed] [Google Scholar]
- 49.Niwa, H., K.-I. Yamamura, and J.-I. Mitazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-200. [DOI] [PubMed] [Google Scholar]
- 50.Oda, K., and Y. Ikehara. 1985. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur. J. Biochem. 152:605-609. [DOI] [PubMed] [Google Scholar]
- 51.Oda, K., Y. Koriyama, E. Yamada, and Y. Ikehara. 1986. Effects of weakly basic amines on proteolytic processing and terminal glycosylation of secretory proteins in cultured rat hepatocytes. Biochem. J. 240:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi, Y., T. Shioda, K. Nakayama, S. Iwata, B. Gotoh, M. Hamaguchi, and Y. Nagai. 1994. A furin-defective cell line is able to process correctly the gp160 of human immunodeficiency virus type 1. J. Virol. 68:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Sullivan, J. D., A. M. Allworth, D. L. Paterson, T. M. Snow, R. Boots, L. J. Gleeson, A. R. Gould, A. D. Hyatt, and J. Bradfield. 1997. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 349:93-95. [DOI] [PubMed] [Google Scholar]
- 54.Paterson, R. G., and R. A. Lamb. 1993. The molecular biology of influenza viruses and paramyxoviruses, p. 35-73. In A. Davidson and R. M. Elliott (ed.), Molecular virology: a practical approach. IRL Oxford University Press, Oxford, England.
- 55.Scheid, A., and P. W. Choppin. 1974. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology 57:475-490. [DOI] [PubMed] [Google Scholar]
- 56.Seidah, N. G., and M. Chrétien. 1999. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 848:45-62. [DOI] [PubMed] [Google Scholar]
- 57.Steiner, D. F. 2002. The prohormone convertases and precursor processing in protein biosynthesis, p. 163-198. In R. E. Dalbey and D. S. Sigman (ed.), The enzymes, vol. XXII. Academic Press, San Diego, Calif. [Google Scholar]
- 58.Stieneke-Gröber, A., M. Vey, H. Angliker, E. Shaw, G. Thomas, C. Roberts, H.-D. Klenk, and W. Garten. 1992. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 11:2407-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugrue, R. J., C. Brown, G. Brown, J. Aitken, and H. W. McL. Rixon. 2001. Furin cleavage of the respiratory syncytial virus fusion protein is not a requirement for its transport to the surface of virus-infected cells. J. Gen. Virol. 82:1375-1386. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi, S., K. Kasai, K. Hatsuzawa, N. Kitamura, Y. Misumi, Y. Ikehara, K. Murakami, and K. Nakayama. 1993. A mutation of furin causes the lack of precursor-processing activity in human colon carcinoma LoVo cells. Biochem. Biophys. Res. Commun. 195:1019-1026. [DOI] [PubMed] [Google Scholar]
- 61.Tamin, A., B. H. Harcourt, T. G. Ksiazek, P. E. Rollin, W. J. Bellini, and P. A. Rota. 2002. Functional properties of the fusion and attachment glycoproteins of Nipah virus. Virology 296:190-200. [DOI] [PubMed] [Google Scholar]
- 62.Thomas, G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3:753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vey, M., W. Schäfer, B. Reis, R. Ohuchi, W. Britt, W. Garten, H. D. Klenk, and K. Radsak. 1995. Proteolytic processing of human cytomegalovirus glycoportein B (gpUL55) is mediated by the human endoprotease furin. Virology 206:746-749. [DOI] [PubMed] [Google Scholar]
- 64.Vincent, M. J., A. J. Sanchez, B. R. Erickson, A. Basak, M. Chrétien, N. G. Seidah, and S. T. Nichol. 2003. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J. Virol. 77:8640-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volchkov, V. E., H. Feldmann, V. A. Volchkova, and H.-D. Klenk. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 95:5762-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vollenweider, F., S. Benjsnnet, E. Decroly, D. Savaria, C. Lazure, G. Thomas, M. Chretien, and N. G. Seidah. 1996. Comparative cellular processing of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp160 by the mammalian subtilisin/kexin-like convertases. Biochem. J. 314:521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, L.-F., M. Yu, E. Hansson, L. I. Pritchard, B. J. Shiell, W. P. Michalski, and B. T. Eaton. 2000. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J. Virol. 74:9972-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willey, R. L., J. S. Bonifacino, B. J. Potts, M. A. Martin, and R. D. Klausner. 1988. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc. Natl. Acad. Sci. USA 85:9580-9584. [DOI] [PMC free article] [PubMed] [Google Scholar]