Figure 4.
Membrane Binding by CHMP7 Is Essential for ESCRT-III-Dependent NE Reformation during Mitotic Exit
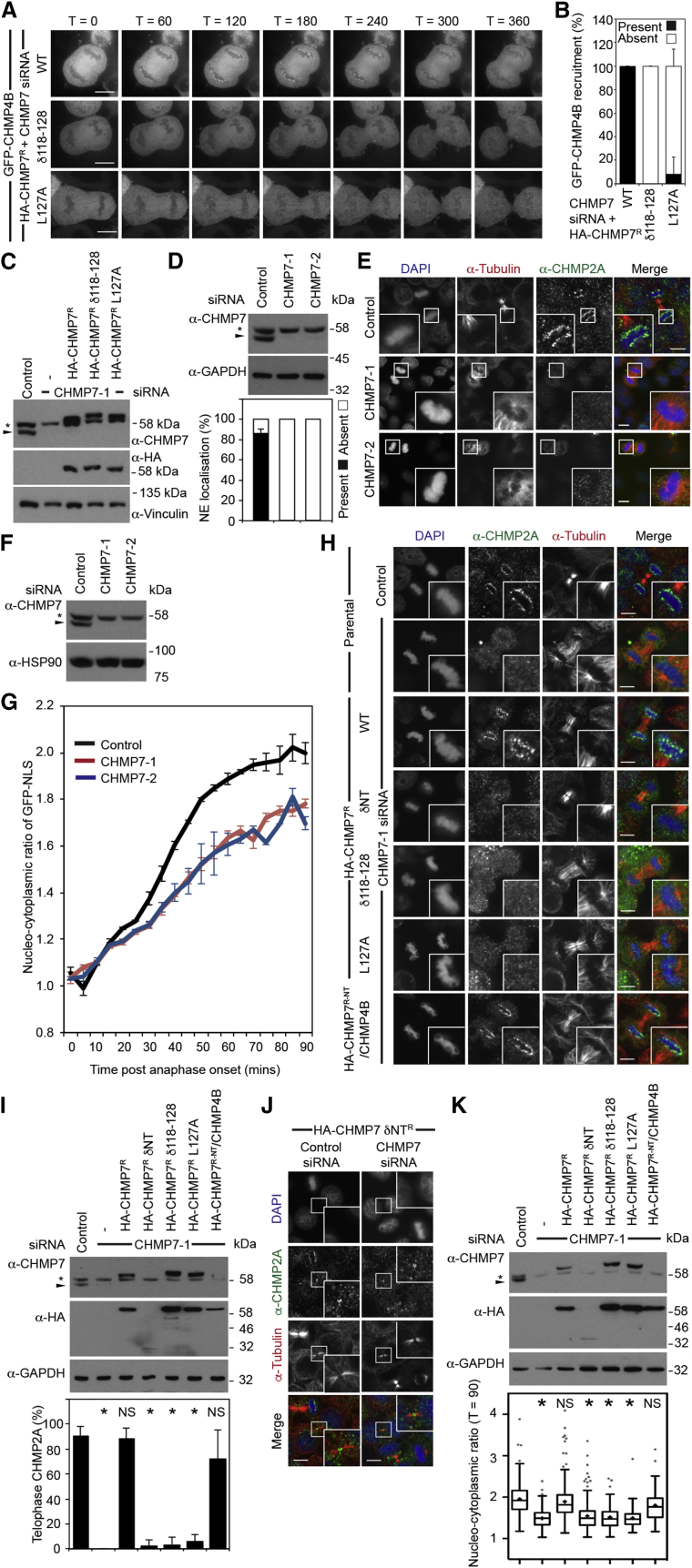
(A) siRNA-transfected HeLa cells stably expressing both GFP-CHMP4B and the indicated HA-CHMP7R proteins were imaged live (time interval in seconds).
(B) Quantification of NE enrichment of GFP-CHMP4B from (A) (mean ± SD; CHMP7 siRNA + HA-CHMP7R, 23/23, N = 4; CHMP7 siRNA and HA-CHMP7R δ118–128, 0/15, N = 3; CHMP7 siRNA and HA-CHMP7R L127A, 1/16, N = 3).
(C) Resolved lysates of cells from (A) were examined by western blotting with anti-CHMP7, anti-HA, or anti-Vinculin.
(D and E) siRNA-transfected HeLa cells were fixed; stained with anti-tubulin, anti-CHMP2A, and DAPI; and examined by immunofluorescence (E) or resolved and examined by western blotting with anti-CHMP7 or anti-GAPDH (D). Assembly of CHMP2A at the telophase NE was quantified (D, mean ± SD; n = 40, N = 2; p = 0.0008, calculated by two-tailed Student’s t test).
(F and G) siRNA-transfected HeLa cells stably expressing GFP-NLS and H2B-mCh were analyzed by western blotting with anti-CHMP7 and anti-HSP90 (F) or were imaged live and the degree of nucleocytoplasmic compartmentalization was calculated at the indicated time points (G, Mean ± SEM; control, N = 4, n = 40; CHMP7 siRNA-1, N = 3, p = 0.010, n = 28; CHMP7 siRNA-2, N = 3, n = 28, p = 0.003). Significance was calculated after 90 min using one-way ANOVA with Dunnett’s multiple comparison test.
(H and I) HeLa cells stably expressing the indicated HA-tagged, siRNA-resistant CHMP7 proteins were transfected with control or CHMP7-targeting siRNA and fixed; stained with anti-tubulin, anti-CHMP2A, and DAPI; and examined by immunofluorescence (H) or resolved and analyzed by western blotting with anti-CHMP7, anti-HA, and anti-GAPDH (I). Cells from (H) were quantified (I, mean ± SD; control, N = 5, n = 80; CHMP7 siRNA, N = 4, n = 34, p < 0.001; CHMP7 siRNA and HA-CHMP7R, N = 4, n = 52, not significant [NS, p = 0.995]; CHMP7 siRNA and HA-CHMP7R δNT, N = 4, n = 40, p < 0.001; CHMP7 siRNA and HA-CHMP7R δ118–128, N = 3, n = 30, p < 0.001; CHMP7 siRNA and HA-CHMP7R L127A, N = 3, n = 32, p < 0.001; CHMP7 siRNA and HA-CHMP7R-NT/CHMP4B, N = 3, n = 31, NS [p = 0.055]). Significance was calculated using one-way ANOVA with Dunnett’s multiple comparison test (∗, significant).
(J) HeLa cells stably expressing HA-CHMP7R δNT were transfected with control or CHMP7-targeting siRNA; fixed; stained with anti-tubulin, anti-CHMP2A, and DAPI; and examined by immunofluorescence. Midbody localization of CHMP2A was observed in 30/30 cases (Ctrl) and 29/30 cases (CHMP7 siRNA) (N = 3).
(K) siRNA-transfected HeLa cells stably expressing GFP-NLS, H2B-mCh, and the indicated HA-tagged siRNA-resistant CHMP7 proteins were examined by western blotting with anti-CHMP7, anti-HA, or anti-GAPDH or were imaged live and the degree of nucleocytoplasmic compartmentalization was calculated 90 min post-anaphase onset (mean ± SEM; control, 1.93 ± 0.04, N = 9, n = 236; CHMP7 siRNA, 1.51 ± 0.03, N = 9, n = 252, p < 0.0001; CHMP7 siRNA and HA-CHMP7R, 1.88 ± 0.05, N = 8, n = 257, NS [p = 0.8909]; CHMP7 siRNA and HA-CHMP7R δNT, 1.54 ± 0.08, N = 4, n = 207, p = < 0.0001; CHMP7 siRNA and HA-CHMP7R δ118–128, 1.53 ± 0.1, N = 3, n = 101, p = 0.0003; CHMP7 siRNA and HA-CHMP7R L127A, 1.50 ± 0.08, N = 3, n = 101, p = 0.0001; CHMP7 siRNA and HA-CHMP7R-NT/CHMP4B, 1.73 ± 0.1, N = 3, n = 76, NS [p = 0.1556]). Statistical significance was calculated using one-way ANOVA with Dunnett’s multiple comparison test from experimental means (N); ∗, significant. Tukey whiskers and mean (+) are displayed.
In all micrographs, the scale bar represents 10 μm.