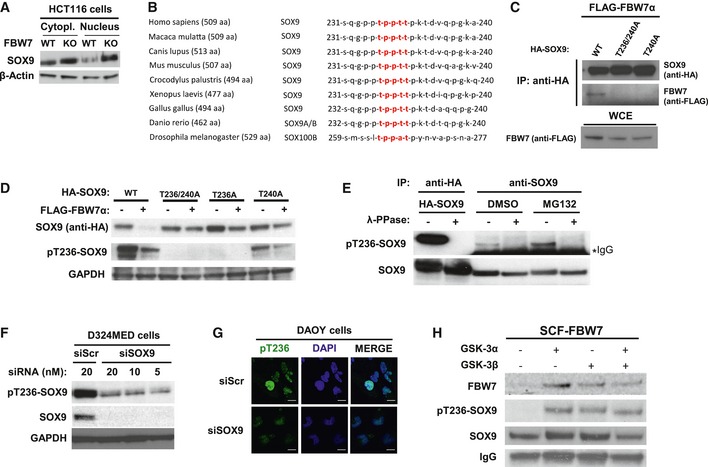
Western blotting of total SOX9 protein levels in the cytoplasmic and nuclear fractions of HCT116‐FBW7 WT versus KO cells. The β‐actin was used as a loading control.
General evolutionary conservation for SOX9 amino acid sequence surrounding the human CPD motif (highlighted in red) of threonine 236–240 across species.
Western blotting of FLAG‐FBW7α eluted from the immunoprecipitated HA‐SOX9 wild‐type (WT) or CPD mutants (‐T236/240A and ‐T240A). The HA‐SOX9‐WT and the CPD mutant constructs were transiently co‐expressed for 24 h with FLAG‐FBW7α in HEK293 prior to immunoprecipitation with anti‐HA antibody. Equal protein expression of FBW7α across the HEK293 cells transfected with different SOX9 constructs was assessed by immunoblotting of the whole‐cell extract.
Co‐expression of FBW7α with HA‐SOX9 WT or various other CPD mutant constructs (‐T236/240A, ‐T236A, or ‐T240A) in HEK293 cells. Whole‐cell lysates were collected 24 h following transfection for Western blotting of the total exogenous and the phosphorylated SOX9 proteins using anti‐HA and our pT236‐SOX9 antibody, respectively. Immunoblot of GAPDH protein was used to indicate protein loading in each lane.
Detection of both exogenous and endogenous phosphorylated SOX9 protein from SOX9 immunoprecipitates. HA‐SOX9‐transfected or non‐transfected HEK293 cells were used as sources for exogenous and endogenous SOX9 protein, respectively. Following SOX9 immunoprecipitation with either anti‐HA (for exogenous) or anti‐SOX9 (for endogenous) antibody, the resulting immunoprecipitates were divided and either treated with λ‐phosphatase or left untreated prior to gel electrophoresis and immunoblotting with pT236‐SOX9 antibody. The SOX9 protein blot shows the total protein level present in each sample. Treatment of HEK293 with proteasome inhibitor MG132 (10 μM) increased the level of phosphorylated SOX9.
Immunoblots of endogenous pT236 and total SOX9 protein 24 h following transfection of D324MED medulloblastoma cell line with either non‐targeting scramble RNA (siScr) or increasing concentrations of siRNA against SOX9. GAPDH protein was used to indicated protein loading for each sample
Representative immunofluorescence staining depicting high intensity of pT236‐SOX9 (Alexa Fluor 488; green) staining in the nucleus (counterstained with DAPI; blue) in Daoy medulloblastoma cells. Transfection of Daoy cells with 20 nM siSOX9 depleted the nuclear staining of pT236‐SOX9. Images were taken using a 40× objective. Scale bar: 20 μm.
Bead‐immobilized IVT HA‐SOX9 WT were subjected to in vitro kinase reaction with 1 unit of recombinant active GSK3α, GSK3β, or their combination (i.e., 0.5 unit for each isoform) for 90 min at 37°C prior to elution and gel electrophoresis. The SOX9 blot shows total SOX9 protein eluted from the beads from each in vitro kinase reaction.