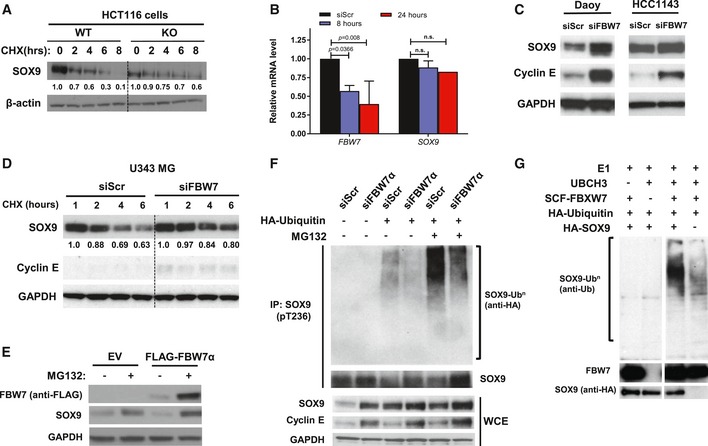
Endogenous SOX9 protein turnover in HCT116‐FBW7 WT and KO cells over the course of 8 h following the addition of 100 ng/ml cycloheximide. β‐Actin indicates total protein loading for each sample.
Quantitative PCR analysis of FBW7 and SOX9 mRNA transcripts in Daoy at 8 and 24 h following transfection with 20 nM siFBW7. The FBW7 and SOX9 mRNAs were adjusted to the B2M mRNA prior to being expressed relative to the siScr control. Data are expressed as mean + standard deviation from two independent experiments, with statistical significance determined by multiple‐comparison two‐way ANOVA with Bonferroni's post‐test.
Immunoblots of endogenous SOX9 protein in medulloblastoma cell line Daoy and breast cancer cell line HCC1143 following depletion of FBW7 by RNAi (20 nM) for 48 h. Accumulation of cyclin E is used to assess the efficiency of FBW7 knockdown. GAPDH immunoblot is shown as a loading control.
Cycloheximide chase of endogenous SOX9 protein over the course of 6 h in glioma cell line U343MG. The cells were transfected with either non‐targeting (siScr) or FBW7‐specific siRNA for 72 h prior to experiments. Immunoblots of cyclin E, established SCFFBW7 substrate, indicated the efficacy of siFBW, while GAPDH protein was used as total protein loading control for each sample.
Western blotting of endogenous SOX9 protein level upon treatment with 10 μM MG132. HEK293 cells were transfected with 1 μg FLAG‐FBW7α for 24 h prior to treatment with the proteasome inhibitor. Whole‐cell lysates were collected 4 h following MG132 treatment for gel electrophoresis and immunoblotting. GAPDH protein was immunoblotted to indicate total protein loading for each sample.
RNAi depletion of FBW7α decreased endogenous SOX9 ubiquitylation in HEK293. The cells were transfected with either scramble (siScr) or siFBW7α for 72 h prior to assessment of endogenous SOX9 ubiquitylation in the absence or presence of MG132. Endogenous SOX9 protein was immunoprecipitated under denaturing condition (1% SDS) from the whole‐cell lysate using the pT236‐SOX9 antibody and eluted as described in
Materials and Methods. Total SOX9, cyclin E, and GAPDH proteins in the whole‐cell lysate were immunoblotted.
Reconstitution of ubiquitylation reaction in vitro using bead‐immobilized IVT HA‐SOX9‐WT and recombinant, active human SCFFBW7α. Reaction mixture lacking the UbcH3, SCFFBW7α, and IVT HA‐SOX9‐WT served as control for the experiments. Ubiquitylation was assessed following elution of IVT HA‐SOX9‐WT from the bead. Immunoblots of SOX9 and FBW7 proteins present in the eluted fraction are shown.