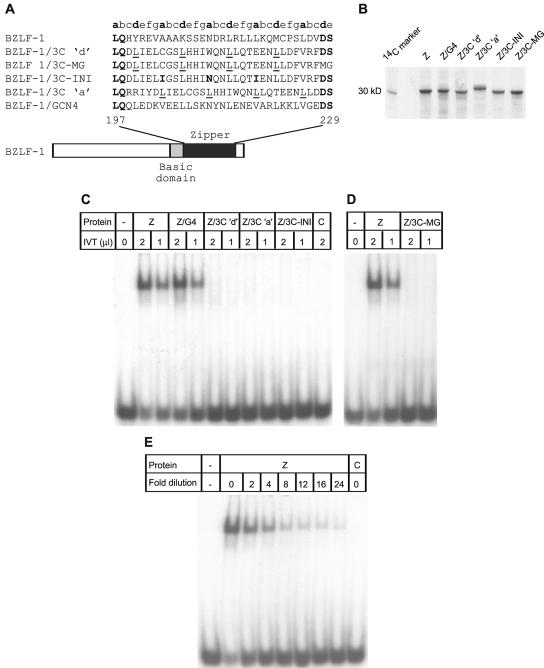
FIG. 5.
Domain-swapping experiments. (A) Diagram showing the hybrid proteins used. (B) SDS-PAGE of the [35S]methionine-labeled proteins used in EMSAs. Equal amounts of each protein were loaded on the gel. BZLF-1/3C ‘a’ consistently ran more slowly in SDS-PAGE, presumably as a result of differences in its amino acid composition. Intensity differences reflect the fact that wild-type BZLF-1 and BZLF-1/3C-MG contain three methionine residues and all other proteins contain two methionine residues. (C) EMSA with in vitro-translated (IVT) wild-type BZLF-1 (Z) and hybrid proteins. Control lanes (C) contained no RNA in vitro-translated controls. (D) EMSA with in vitro-translated BZLF-1/3C-MG. (E) EMSA with a series of dilutions of BZLF-1 protein. Lanes 1 and 9 contain 2 μl of undiluted BZLF-1 and 2 μl of the no-RNA in vitro-translated control, respectively.