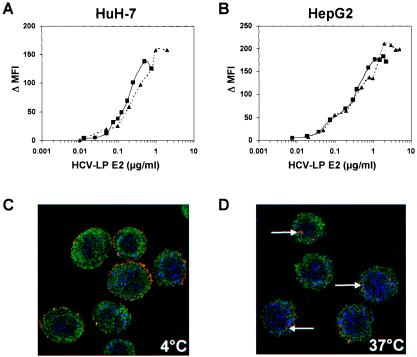
FIG. 1.
Concentration-dependent and saturable binding of HCV-LPs to and entry into human hepatoma cells. (A and B) Binding of HCV-LPs to target cells is concentration-dependent and saturable. HuH-7 (A) and HepG2 (B) cells were incubated with increasing concentrations of HCV-LPs of genotype 1a (triangles), as well as genotype 1b (squares), and particle binding was analyzed by flow cytometry as described in Materials and Methods. On the y axis, net MFI values for each HCV-LP E2 concentration were calculated by subtracting the MFI of the negative control with anti-E2 and PE-conjugated anti-mouse IgG antibodies from that obtained with the respective HCV-LP concentration (x axis). (C and D) Analysis of HCV-LP entry into target cells using anti-E2-specific immunofluorescence and cross section laser scanning confocal microscopy. (C) Cellular binding of HCV-LP was assessed by incubation of HuH-7 cells with HCV-LPs (genotype 1a) at 4°C for 40 min. (D) For temperature-dependent HCV-LP entry, cells were incubated for an additional 60 min at 37°C. After removal of nonbound HCV-LPs by washing the cells with ice-cold PBS, the cells were added to poly-l-lysine-coated cover slides, fixed, permeabilized, and stained for HCV-LPs using anti-E2 and Cy3-conjugated anti-mouse IgG antibodies (arrows). For the costaining of the cytoskeleton, cells were coincubated with a rabbit anti-actin and FITC-conjugated anti-rabbit IgG antibody. For costaining of the nucleus, the cells were incubated with DRAQ-5, a highly permeable DNA-interactive agent.